Fosaprepitant
Fosaprepitant (Emend for Injection (US), Ivemend (EU)) is an antiemetic medication,[3] administered intravenously. It is a prodrug of aprepitant.
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Emend, Ivemend |
| AHFS/Drugs.com | Professional Drug Facts |
| MedlinePlus | a604003 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Protein binding | >95% (aprepitant) |
| Metabolism | To aprepitant |
| Elimination half-life | 9 to 13 hours (aprepitant) |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
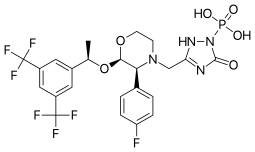
| Formula | C23H22F7N4O6P |
| Molar mass | 614.414 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Fosaprepitant was developed by Merck & Co. and was approved for medical use in the United States,[4] and in the European Union in January 2008.[2]
References
- "Emend- fosaprepitant dimeglumine injection, powder, lyophilized, for solution". DailyMed. 2 May 2022. Archived from the original on 1 December 2021. Retrieved 27 September 2022.
- "Ivemend EPAR". European Medicines Agency. 17 September 2018. Archived from the original on 31 December 2021. Retrieved 27 September 2022.
- Garnock-Jones KP (September 2016). "Fosaprepitant Dimeglumine: A Review in the Prevention of Nausea and Vomiting Associated with Chemotherapy". Drugs. 76 (14): 1365–72. doi:10.1007/s40265-016-0627-7. PMID 27510503. S2CID 30018182.
- "Drugs.com, FDA Approves Emend (fosaprepitant dimeglumine) for Injection, Merck's New Intravenous Therapy, for Use in Combination with Other Antiemetics for Prevention of Nausea and Vomiting Caused by Chemotherapy". Archived from the original on 2008-04-09. Retrieved 2008-03-15.
External links
- "Fosaprepitant dimeglumine". Drug Information Portal. U.S. National Library of Medicine.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.