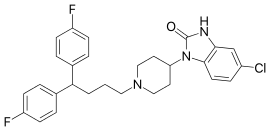
Clopimozide
Clopimozide (R-29,764) is a typical antipsychotic drug of the diphenylbutylpiperidine class.[1][2] It is very potent and has an extremely long duration of action, lasting at least one week with a single dose.[3][4][5] It was developed by Janssen Pharmaceutica but was never marketed.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H28ClF2N3O |
| Molar mass | 496.00 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Synthesis
- [53786-28-0] also used for: Domperidone, Halopemide & Axamozide.
- [3312-04-7] is also used for: Amperozide, Fluspirilene, Pimozide, Penfluridol, Lidoflazine & R 1624.
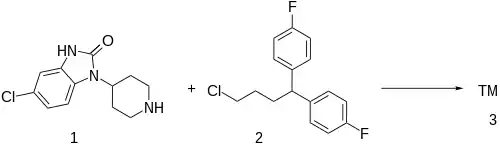
Patent (Ex VIII):[6]
The alkylation between 5-Chloro-1-(4-Piperidyl)-2-Benzimidazolinone [53786-28-0] (1) and 1,1'-(4-Chlorobutylidene)bis(4-fluorobenzene) [3312-04-7] (2) gives clopimozide (3).
References
- De Cuyper HJ, Van Praag HM, Mulder WR (May 1979). "Therapeutical significance of clopimozide in the treatment of chronic psychotic patients". Acta Psychiatrica Scandinavica. 59 (5): 561–74. doi:10.1111/j.1600-0447.1979.tb00256.x. PMID 37697. S2CID 30954603.
- Knapen J, Bollen J, Brugmanns J, Rombaut N (1976). "[Treatment of chronic psychoses with oral clopimozide]". Acta Psychiatrica Belgica (in French). 76 (4): 644–57. PMID 798469.
- Janssen PA, Niemegeers CJ, Schellekens KH, Lenaerts FM, Wauquier A (August 1975). "Clopimozide (R 29 764), a new highly potent and orally long-acting neuroleptic of the diphenylbutylpiperidine series". Arzneimittel-Forschung. 25 (8): 1287–94. PMID 1242360.
- Floru L, Tegeler J (1978). "Clinical experiments with the new oral long-acting neuroleptic clopimozide (R 29 764)". Arzneimittel-Forschung. 28 (2): 341–4. PMID 25071.
- Bobon J, Parent M, Toussaint C, Pinchard A (1976). "[Long-acting neuroleptics. IV. Preliminary study of clopimozide (R 29764)]". Acta Psychiatrica Belgica (in French). 76 (1): 138–43. PMID 970182.
- Paul Adriaan Jan Janssen, 3 weitere », U.S. Patent 3,989,707 (1976 to Janssen Pharmaceutica N.V.).
| Typical |
|
|---|---|
| Disputed | |
| Atypical |
|
| Others |
|
| |
| D1-like |
| ||||||
|---|---|---|---|---|---|---|---|
| D2-like |
| ||||||
| |||||||
Histamine receptor modulators | |||||
|---|---|---|---|---|---|
| H1 |
| ||||
| H2 |
| ||||
| H3 |
| ||||
| H4 |
| ||||
| |||||
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.