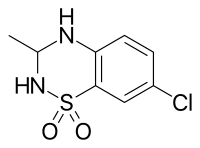
IDRA-21
IDRA-21 is a positive allosteric modulator of the AMPA receptor and a benzothiadiazine derivative. It is a chiral molecule, with (+)-IDRA-21 being the active form.[1]
 | |
| Legal status | |
|---|---|
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C8H9ClN2O2S |
| Molar mass | 232.68 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
IDRA-21 shows nootropic effects in animal studies, significantly improving learning and memory. It is around 10–30 times more potent than aniracetam in reversing cognitive deficits induced by alprazolam or scopolamine,[2][3] and produces sustained effects lasting for up to 48 hours after a single dose.[4] The mechanism for this action is thought to be through promoting the induction of long-term potentiation between synapses in the brain.[5]
IDRA-21 may not produce neurotoxicity under normal conditions,[6] although it may worsen neuronal damage following global ischemia after stroke or seizures.[7]
In comparison to the ampakines or benzoylpiperidine-derived AMPA receptor potentiators, IDRA-21 was more potent than CX-516, but less potent than CX-546.[8] Newer benzothiadiazide derivatives with greatly increased potency compared to IDRA-21 have been developed,[9][10] but these have not been researched to the same extent, with the benzoylpiperidine and benzoylpyrrolidine CX-series of drugs being favoured for clinical development, most likely due to more favourable toxicity profiles at high doses.[11]
See also
References
- Uzunov DP, Zivkovich I, Pirkle WH, Costa E, Guidotti A (August 1995). "Enantiomeric resolution with a new chiral stationary phase of 7-chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide, a cognition-enhancing benzothiadiazine derivative". Journal of Pharmaceutical Sciences. 84 (8): 937–42. doi:10.1002/jps.2600840807. PMID 7500277.
- Thompson DM, Guidotti A, DiBella M, Costa E (August 1995). "7-Chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide (IDRA 21), a congener of aniracetam, potently abates pharmacologically induced cognitive impairments in patas monkeys". Proceedings of the National Academy of Sciences of the United States of America. 92 (17): 7667–71. Bibcode:1995PNAS...92.7667T. doi:10.1073/pnas.92.17.7667. PMC 41206. PMID 7644474.
- Zivkovic I, Thompson DM, Bertolino M, Uzunov D, DiBella M, Costa E, Guidotti A (January 1995). "7-Chloro-3-methyl-3-4-dihydro-2H-1,2,4 benzothiadiazine S,S-dioxide (IDRA 21): a benzothiadiazine derivative that enhances cognition by attenuating DL-alpha-amino-2,3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid (AMPA) receptor desensitization". The Journal of Pharmacology and Experimental Therapeutics. 272 (1): 300–9. PMID 7815345.
- Buccafusco JJ, Weiser T, Winter K, Klinder K, Terry AV (January 2004). "The effects of IDRA 21, a positive modulator of the AMPA receptor, on delayed matching performance by young and aged rhesus monkeys". Neuropharmacology. 46 (1): 10–22. doi:10.1016/j.neuropharm.2003.07.002. PMID 14654093. S2CID 26443642.
- Arai A, Guidotti A, Costa E, Lynch G (September 1996). "Effect of the AMPA receptor modulator IDRA 21 on LTP in hippocampal slices". NeuroReport. 7 (13): 2211–5. doi:10.1097/00001756-199609020-00031. PMID 8930991. S2CID 35888339.
- Impagnatiello F, Oberto A, Longone P, Costa E, Guidotti A (June 1997). "7-Chloro-3-methyl-3,4-dihydro-2H-1,2,4-benzothiadiazine S,S-dioxide: a partial modulator of AMPA receptor desensitization devoid of neurotoxicity". Proceedings of the National Academy of Sciences of the United States of America. 94 (13): 7053–8. Bibcode:1997PNAS...94.7053I. doi:10.1073/pnas.94.13.7053. PMC 21283. PMID 9192690.
- Yamada KA, Covey DF, Hsu CY, Hu R, Hu Y, He YY (May 1998). "The diazoxide derivative IDRA 21 enhances ischemic hippocampal neuron injury". Annals of Neurology. 43 (5): 664–9. doi:10.1002/ana.410430517. PMID 9585363. S2CID 39977647.
- Nagarajan N, Quast C, Boxall AR, Shahid M, Rosenmund C (November 2001). "Mechanism and impact of allosteric AMPA receptor modulation by the ampakine CX546". Neuropharmacology. 41 (6): 650–63. doi:10.1016/S0028-3908(01)00133-2. PMID 11640919. S2CID 7796112.
- Phillips D, Sonnenberg J, Arai AC, Vaswani R, Krutzik PO, Kleisli T, et al. (May 2002). "5'-alkyl-benzothiadiazides: a new subgroup of AMPA receptor modulators with improved affinity". Bioorganic & Medicinal Chemistry. 10 (5): 1229–48. CiteSeerX 10.1.1.113.7845. doi:10.1016/S0968-0896(01)00405-9. PMID 11886787.
- Arai AC, Xia YF, Kessler M, Phillips D, Chamberlin R, Granger R, Lynch G (September 2002). "Effects of 5'-alkyl-benzothiadiazides on (R,S)-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor biophysics and synaptic responses". Molecular Pharmacology. 62 (3): 566–77. doi:10.1124/mol.62.3.566. PMID 12181433.
- Black MD (April 2005). "Therapeutic potential of positive AMPA modulators and their relationship to AMPA receptor subunits. A review of preclinical data". Psychopharmacology. 179 (1): 154–63. doi:10.1007/s00213-004-2065-6. PMID 15672275. S2CID 5869366.