SAGE-718
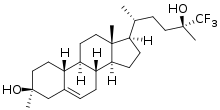
SAGE-718 is experimental drug being investigated for the treatment of neurological disorders and cognitive impairment.[1] It acts as a positive allosteric modulator of the NMDA receptor, whose activity is essential for learning, memory, and cognition.[2] SAGE-718 is an analog of the neurosteroid 24S-hydroxycholesterol.[2]
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C26H41F3O2 |
| Molar mass | 442.607 g·mol−1 |
As of 2022, SAGE-718 is in Phase II clinical trials[2] for Alzheimer's disease,[3][4][5] Parkinson's disease, and Huntington's disease.[6][7]
References
- Hill MD, Blanco MJ, Salituro FG, Bai Z, Beckley JT, Ackley MA, et al. (July 2022). "SAGE-718: A First-in-Class N-Methyl-d-Aspartate Receptor Positive Allosteric Modulator for the Potential Treatment of Cognitive Impairment". Journal of Medicinal Chemistry. doi:10.1021/acs.jmedchem.2c00313. PMID 35785990. S2CID 250250073.
- "SAGE-718". ALZFORUM.
- Shapiro L. "#AAN2022 – SAGE-718 May Help With Cognitive Function in Alzheimer's". BioNews, Inc.
- "SAGE-718 in Patients With Mild Cognitive Impairment or Mild Dementia Due to Alzheimer's Disease: Results From the Phase 2 LUMINARY Study" (PDF). American Academy of Neurology (AAN) 74th Annual Meeting Abstract.
- Castañeda R (16 November 2021). "Regulatory roundup: Sage Alzheimer's asset likely to progress after Phase IIa completion". Clinical Trials Arena.
- "Sage Therapeutics Provides Important Update On The Clinical Development Program For Sage's Investigational Drug, SAGE-718". Huntington's Disease Society of America.
- Wexler M (21 September 2021). "SAGE-718 on FDA Fast Track as Potential Huntington's Disease Therapy". BioNews, Inc.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.