Vonoprazan
Vonoprazan, sold under the brand name Takecab among others, is a first-in-class potassium-competitive acid blocker medication. It was approved in the Japanese market in February 2015.[1]
 | |
| Clinical data | |
|---|---|
| Trade names | Takecab |
| License data |
|
| Drug class | Potassium-competitive acid blocker |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Unknown |
| Protein binding | 80% |
| Metabolism | Liver, by cytochrome P450 (3A4, 2B6, 2C19, 2D6) |
| Elimination half-life | 7.7 h |
| Duration of action | > 24 h |
| Excretion | Kidney |
| Identifiers | |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL |
|
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
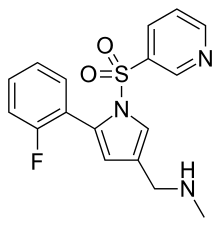
| Formula | C17H16FN3O2S |
| Molar mass | 345.39 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Vonoprazan is used in form of the fumarate for the treatment of gastroduodenal ulcer (including some drug-induced peptic ulcers) and reflux esophagitis, and can be combined with antibiotics for the eradication of Helicobacter pylori.[2]
Co-packaged combinations of vonoprazan with amoxicillin and vonoprazan with amoxicillin and clarithromycin were approved for medical use in the United States in May 2022.[3][4]
A decision on the approval of vonoprazan for treatment of erosive esophagitis in the United States is expected in January 2023.[5]
Society and culture
Names
Vonoprazan is the international nonproprietary name (INN).[6]
References
- Garnock-Jones KP (2015). "Vonoprazan: first global approval". Drugs. 75 (4): 439–43. doi:10.1007/s40265-015-0368-z. PMID 25744862. S2CID 43293048.
- Echizen H (2016). "The First-in-Class Potassium-Competitive Acid Blocker, Vonoprazan Fumarate: Pharmacokinetic and Pharmacodynamic Considerations". Clin Pharmacokinet. 55 (4): 409–18. doi:10.1007/s40262-015-0326-7. PMID 26369775. S2CID 5984975.
- "Voquezna Dual Pak- vonoprazan fumarate and amoxicillin kit Voquezna Triple Pak- vonoprazan fumarate, amoxicillin and clarithromycin kit". DailyMed. 21 June 2022. Archived from the original on 3 July 2022. Retrieved 3 July 2022.
- "Phathom Pharmaceuticals Announces FDA Approval of Voquezna Triple Pak (vonoprazan, amoxicillin, clarithromycin) and Voquezna Dual Pak (vonoprazan, amoxicillin) for the Treatment of H. pylori Infection in Adults" (Press release). Phathom Pharmaceuticals. 3 May 2022. Archived from the original on 10 May 2022. Retrieved 9 May 2022 – via GlobeNewswire.
- "Phathom Pharmaceuticals Announces FDA Acceptance for Filing of Vonoprazan NDA for the Treatment of Erosive Esophagitis" (Press release). Phathom Pharmaceuticals. 25 May 2022. Retrieved 15 September 2022.
- World Health Organization (2012). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 68". WHO Drug Information. 26 (3). hdl:10665/109813.