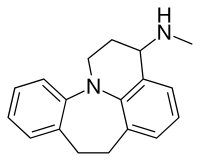
Ciclopramine
Ciclopramine is a tetracyclic antidepressant (TeCA) that was never marketed.[1][2]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C18H20N2 |
| Molar mass | 264.372 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
The structure is best compared to desipramine, where the sidechain has been abridged to the tricyclic dibenzazepine ring.
Synthesis
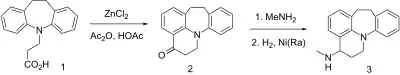
Patent:[3]
The reaction of 3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)propionic acid, CID:22390899 (1) with lewis acid in a mixture of acetic anhydride and acetic acid led to the characteristic Haworth cyclization reaction to give 1,2,7,8-tetrahydro-3H-quino[1,8-ab][1]benzazepin-3-one, CID:21853518 (2). The condensation with methylamine gives a Schiff-base; the reduction to the secondary amine compound completed the synthesis of ciclopramine (3).
References
- Triggle DJ (1997). Dictionary of pharmacological agents. London: Chapman & Hall. ISBN 0-412-46630-9.
- Saletu B (1982). "Pharmaco-EEG profiles of typical and atypical antidepressants". Advances in Biochemical Psychopharmacology. 32: 257–68. PMID 7090895.
- DE2004818 idem G Hackmack & H Menge, U.S. Patent 3,830,818 (1974 to Takeda GmbH)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.