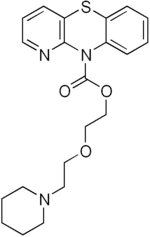
Pipazetate
Pipazetate (INN) (brand names Dipect, Lenopect, Selvigon, Theratuss, Toraxan), or pipazethate (USAN), is a pyridobenzthiazine cough suppressant, closely related to the phenothiazine class.[1][2] It binds to the sigma-1 receptor with an IC50 value of 190 nM.[3] It also has local anesthetic action, and in large doses can produce seizures.[4]
 | |
| Clinical data | |
|---|---|
| Trade names | Dipect, Lenopect, Selvignon, Selvigon, Theratuss, Toraxan |
| Other names | Pipazethate; D-254; LG-254; SKF-70230A; SQ-15874 |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.016.826 |
| Chemical and physical data | |
| Formula | C21H25N3O3S |
| Molar mass | 399.51 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
As the brand name Theratuss, it was approved by the FDA in 1962, on evidence of safety only. It was withdrawn from the US market in 1972 when the manufacturer, E.R. Squibb and Sons, failed to produce evidence of efficacy.[5] Clinical studies showed that it did not decrease cough frequency at recommended dosages.[6]
Side effects, which are infrequent, include nausea, vomiting, drowsiness, fatigue, rash, and tachycardia.[6]
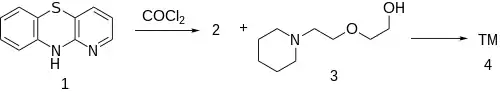
Synthesis
Note: Same Pyridobenzthiazine used for Prothipendyl & Isothipendyl.
The reaction of 1-azaphenothiazine [261-96-1] (1) with phosgene gives 1-azaphenothiazine-10-carbonyl chloride [94231-78-4] (2). The reaction of this reactive intermediate with 2-[2-(piperidyl)ethoxy]ethanol [3603-43-8] (3) gives the ester, thus completing the synthesis of Pipazethate (4).
References
- Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 985–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 1418–. ISBN 978-3-88763-075-1.
- Klein M, Musacchio JM (October 10, 1988). "Dextromethorphan binding sites in the guinea pig brain". Cellular and Molecular Neurobiology. 8 (2): 149–156. doi:10.1007/BF00711241. PMID 3044591. S2CID 33844132.
- Martín, Alfonso Velasco (2004). "Tratamiento sintomático de la tos y del resfriado común". Farmacología clínica y terapéutica médica. p. 259. ISBN 9788448604271.
- Certain Preparations Containing Dihyprylone or Pipazethate Hydrochloride; Notice of Withdrawal of Approval of New-Drug Applications (PDF). Federal Register (Report). Vol. 37. August 5, 1972. p. 15887. FDC–D–458.
- Council on Drugs (1971). AMA Drug Evaluations (Report). Chicago: American Medical Association. p. 360–3. LCCN 75147249. Retrieved April 5, 2021.
- Schuler, Wilhelm A.; Klebe, Hans; Schlichtegroll, Ansgar V. (1964). "Synthesen von 4-Aza-phenothiazinen, II. Derivate der 4-Aza-phenothiazin-10-carbonsäure". Justus Liebigs Annalen der Chemie. 673 (1): 102–112. doi:10.1002/jlac.19646730114.
- Schuler Wilhelm Alfons, U.S. Patent 2,989,529 (1961 to Degussa).
- Bernd Dr. Lehmann & Bernhard Petrat, EP 0527298 (1997 to Meda Pharma GmbH and Co KG).