Cinepazide
Cinepazide or cinepazide maleate[1] (Kelinao or Anjieli in China[2][3]) is a vasodilator used in China for the treatment of cardiovascular and cerebrovascular diseases, and peripheral vascular diseases.[4] It appears to work by potentiating A2 adenosine receptors.[5]
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.739 |
| Chemical and physical data | |
| Formula | C22H31N3O5 |
| Molar mass | 417.49864 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
History
Cinepazide was discovered by scientists at Laboratoires Delalande (now part of Sanofi) in 1969 in an effort to explore useful substituted cinnamoyl-piperazine compounds.[6][7] The drug, in the form of a pill taken orally, was launched by Delalande in 1976 under the tradename Vasodistal, for treatment of heart failure, balance disorders, cerebrovascular disease, and vascular complications of diabetes.[6][8] In 1988 the drug was withdrawn from the market in Spain due to risk of agranulocytosis; other countries where the drug was available added warnings to the label.[9][10] It was withdrawn from the market in France in 1992.[11] The drug had also been marketed in Japan by Daiichi Pharmeceutical Company under the brand name "Brindel"[2] for dementia, but was withdrawn in 1999, following a review by the Japanese regulatory authorities of dementia drugs after a drug, calcium hopantenate, that had been considered the standard of care and against which cinepazide and other dementia drugs had been compared, had failed to demonstrate efficacy in a re-evaluation.[12]
In 2002 Sihuan Pharmaceutical brought an injectable form of the drug to market in China;[13] Sihuan had acquired the drug from a military hospital in China that had developed the formulation.[14] In 2010 it was the highest selling drug in China, with about 1 billion RMB in sales in the 3rd quarter, outselling Plavix in China.[13][3] This made Sihuan Pharm the largest company in China in the cardio-cerebral vascular drug market in 2010.[3] In 2014 it was the tenth highest-selling drug in China.[14]
Synthesis
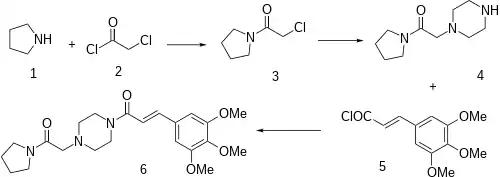
Amide formation between chloroacetyl chloride (1) and pyrrolidine (2) gives 1-(chloroacetyl)pyrrolidine [20266-00-6] (3). Displacement of the remaining halogen by piperazine gives 1-(pyrrolidinocarbonylmethyl)piperazine [39890-45-4] (4). A second Schotten-Baumann reaction this time with 3,4,5-trimethoxycinnamoyl chloride [4521-61-3] (5) completed the synthesis of cinepazide (6).
References
- "Anjieli, Kelinao, cinepazide maleate - Product Profile - BioCentury". www.biocentury.com. Retrieved 2015-08-04.
- Drugs.com Drugs.com International listings for cinepazide Page accessed Aug 3, 2015
- 20 Oct 2010 Sihuan Pharm – China's leading player in cardio-cerebral vascular drug – IPO Report Archived 2015-09-23 at the Wayback Machine
- Sihuan Pharmaceutical Kelinao/Anjieli official site Page accessed Aug 3, 2015
- Ruffolo RR, Hieble JP, Brooks DP, Feuerstein GZ, Nichols AJ (1991). "Drug receptors and control of the cardiovascular system: recent advances". Progress in Drug Research. Fortschritte der Arzneimittelforschung. Progrès des Recherches Pharmaceutiques. 36: 117–360. doi:10.1007/978-3-0348-7136-5_4. ISBN 978-3-0348-7138-9. PMID 1876708.
- Johnson Sun for Guotai Junan International. Sept 28, 2011. Company Report: Sihuan Pharmaceuticals
- Cameron BD, Chasseaud LF, Hawkins DR, Taylor T (July 1976). "The metabolic fate of the coronary vasodilator 4-(3,4,5-Trimethoxycinnamoyl)-1-(N-pyrrolidinocarbonylmethyl)piperazine (cinepazide) in the rat, dog and man". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 6 (7): 441–55. doi:10.3109/00498257609151657. PMID 997590.
- Reactions Weekly 305(1):1. June 1990 Cinepazide-related agranulocytosis
- Laporte JR, Capellà D, Juan J (1990). "Agranulocytosis induced by cinepazide". European Journal of Clinical Pharmacology. 38 (4): 387–8. doi:10.1007/bf00315580. PMID 2344862. S2CID 19427552.
- Department of Economic and Social Affairs of the United Nations Secretariat Consolidated List of Products Whose Consumption and/or Sale Have Been Banned, Withdrawn, Severely Restricted or not Approved by Governments Twelfth Issue: Pharmaceuticals United Nations – New York, 2005
- Sidney M. Wolfe, M.D. for the Public Citizen's Health Research Group. February 2, 1995. Differences in the Number of Drug Safety Withdrawals: United States, United Kingdom, Germany and France 1970-1992
- Takeda M, Tanaka T, Okochi M (August 2011). "New drugs for Alzheimer's disease in Japan". Psychiatry and Clinical Neurosciences. 65 (5): 399–404. doi:10.1111/j.1440-1819.2011.02253.x. PMID 21851448. S2CID 21519637.
- Lefei Sun, Jinsong Du, and Iris Wang for Credit Suisse. October 6, 2011 China Pharma Sector
- Su Zhang for Standard Chartered Bank (HK) Limited. June 27, 2014 China health care: Pharma sector comes of age
- C. Fauran and M. Turin, Chim. Ther., 4, 290 (1969).
- Ohtaka, H., Yoshida, K., Suzuki, K., Shimohara, K., Tajima, S., Ito, K. (1988). "Benzylpiperazine derivatives. VIII. Syntheses, antiulcer and cytoprotective activities of 1-(aminocarbonylalkyl)-4-benzylpiperazine derivatives and related compounds". Chemical and Pharmaceutical Bulletin. 36 (10): 3948–3954. doi:10.1248/cpb.36.3948.
- Yasuo Itho, et al. U.S. Patent 4,478,838 (1984 to Abbott Japan Co Ltd).
- Liu Da, et al. CN 112028856 (2020 to Kamp Pharmaceuticals Co Ltd).