Suloctidil
Suloctidil was a sulfur-containing aminoalcohol that was brought to market in the early 1970s as a vasodilator by Continental Pharma, a Belgian company.[1]: 118–121 [2][3]
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.053.920 |
| Chemical and physical data | |
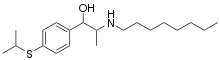
| Formula | C20H35NOS |
| Molar mass | 337.563 g·mol−1 |
| (verify) | |
Continental was bought by Monsanto in 1984, primarily on the promise of sales of suloctidil, which was approved in Europe at the time, but not in the US.[4] However, in 1985 Monsanto halted development and withdrew the drug worldwide following reports of liver toxicity.[5]: 251
References
- Hladovec J (1989). Antithrombotic Drugs in Thrombosis Models. CRC Press. ISBN 9780849351624.
- Roncucci R, Roba J, Lambelin G, Ferenczi M, Blaton V, Vandamme D, Peeters H (March 1975). "Potential antilipolytic activity of suloctidil". Die Naturwissenschaften. 62 (3): 141–2. Bibcode:1975NW.....62..141R. doi:10.1007/bf00623284. PMID 1240601. S2CID 29484315.
- BE granted 739678, Buu-Hoi NP, Lambelin G, Roba J, Jacques G, Gillet C, "1-Subst-phenyl-2-amino-ethanols useful as beta adrenergic agents peripheral vasodilators and hypotensive agents"
- Staff (5 November 1984). "Monsanto's $150 mil. Life Sciences Research Center". The Pink Sheet.
{{cite web}}: CS1 maint: uses authors parameter (link) - "Consolidated List of Products Whose Consumption and/or Sale Have Been Banned, Withdrawn, Severely Restricted or not Approved by Governments Twelfth Issue: Pharmaceuticals" (PDF). Department of Economic and Social Affairs of the United Nations Secretariat. New York: United Nations. 2005.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.