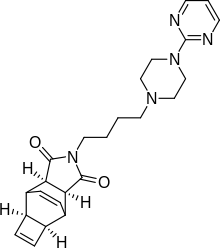
Zalospirone
Zalospirone (WY-47,846) is a selective 5-HT1A partial agonist of the azapirone chemical class.[1][2] It was found to be effective in the treatment of anxiety and depression in clinical trials, but a high proportion of subjects dropped out due to side effects and development was subsequently never completed.[3]
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 1-4 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C24H29N5O2 |
| Molar mass | 419.529 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Zalospirone was patented for the treatment of alcoholism, sleep disorders,Alzheimer's disease:[4]
Synthesis
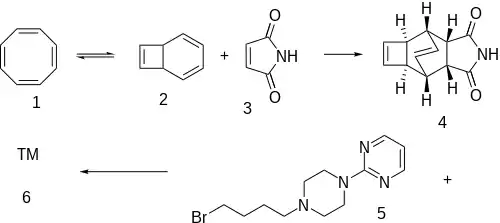
An internal electrocyclic rearrangement means that cyclooctatetraene [629-20-9] (1) is in equilibrium with bicyclo[4.2.0]octa-2,4,7-triene, CID:12676027 (2). Diels-Alder reaction with the very reactive dienophile maleimide [541-59-3] (3) leads to the bridged bicyclic adduct [114298-16-7] (4). Alkylation with [87789-48-8] (5) completed the synthesis of Zalospirone (6).
See also
References
- Gleeson S, Barrett JE (October 1990). "5-HT1A agonist effects on punished responding of squirrel monkeys". Pharmacology, Biochemistry, and Behavior. 37 (2): 335–7. doi:10.1016/0091-3057(90)90344-H. PMID 1981937. S2CID 23488390.
- Singh A, Lucki I (April 1993). "Antidepressant-like activity of compounds with varying efficacy at 5-HT1A receptors". Neuropharmacology. 32 (4): 331–40. doi:10.1016/0028-3908(93)90153-T. PMID 8497336. S2CID 38611829.
- Rickels K, Derivan A, Kunz N, Pallay A, Schweizer E (June 1996). "Zalospirone in major depression: a placebo-controlled multicenter study". Journal of Clinical Psychopharmacology. 16 (3): 212–7. doi:10.1097/00004714-199606000-00004. PMID 8784652.
- Magid A. M. Abou-Gharbia & John A. Moyer, U.S. Patent 5,183,819 (1993 to Wyeth LLC).
- Abou-Gharbia, M., Patel, U. R., Webb, M. B., Moyer, J. A., Andree, T. H., Muth, E. A. (July 1988). "Polycyclic aryl- and heteroarylpiperazinyl imides as 5-HT1A receptor ligands and potential anxiolytic agents: synthesis and structure-activity relationship studies". Journal of Medicinal Chemistry. 31 (7): 1382–1392. doi:10.1021/jm00402a023. ISSN 1520-4804 0022-2623, 1520-4804.
{{cite journal}}: Check|issn=value (help) - Magid A. Abou-Gharbia, U.S. Patent 4,892,943 (1990 to Wyeth LLC).
| 5-HT1AR agonists | |
|---|---|
| GABAAR PAMs |
|
| Gabapentinoids (α2δ VDCC blockers) | |
| Antidepressants |
|
| Sympatholytics (Antiadrenergics) |
|
| Others | |
| |
Serotonin receptor modulators | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-HT1 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT2 |
| ||||||||||||||||||||||||||||||||||||||
| 5-HT3–7 |
| ||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||
| Simple piperazines (no additional rings) |
|
|---|---|
| Phenylpiperazines |
|
| Benzylpiperazines | |
| Diphenylalkylpiperazines (benzhydrylalkylpiperazines) |
|
| Pyrimidinylpiperazines |
|
| Pyridinylpiperazines |
|
| Benzo(iso)thiazolylpiperazines | |
| Tricyclics (piperazine attached via side chain) |
|
| Others/Uncategorized |
|