SB-357134
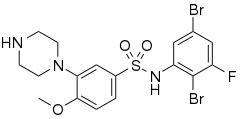
SB-357134 is a drug which is used in scientific research. It acts as a potent, selective and orally active 5-HT6 receptor antagonist.[1] SB-357134 and other 5-HT6 antagonists show nootropic effects in animal studies,[2][3][4] and have been proposed as potential novel treatments for cognitive disorders such as schizophrenia and Alzheimer's disease.
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider |
|
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H18Br2FN3O3S |
| Molar mass | 523.22 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
References
- Bromidge SM, Clarke SE, Gager T, Griffith K, Jeffrey P, Jennings AJ, et al. (January 2001). "Phenyl benzenesulfonamides are novel and selective 5-HT6 antagonists: identification of N-(2,5-dibromo-3-fluorophenyl)-4-methoxy-3-piperazin-1-ylbenzenesulfonamide (SB-357134)". Bioorganic & Medicinal Chemistry Letters. 11 (1): 55–8. doi:10.1016/S0960-894X(00)00597-7. PMID 11140733.
- Rogers DC, Hagan JJ (November 2001). "5-HT6 receptor antagonists enhance retention of a water maze task in the rat". Psychopharmacology. 158 (2): 114–9. doi:10.1007/s002130100840. PMID 11702084. S2CID 29472459.
- Stean TO, Hirst WD, Thomas DR, Price GW, Rogers D, Riley G, et al. (April 2002). "Pharmacological profile of SB-357134: a potent, selective, brain penetrant, and orally active 5-HT(6) receptor antagonist". Pharmacology, Biochemistry, and Behavior. 71 (4): 645–54. doi:10.1016/S0091-3057(01)00742-0. PMID 11888556. S2CID 34925312.
- Perez-García G, Meneses A (July 2005). "Oral administration of the 5-HT6 receptor antagonists SB-357134 and SB-399885 improves memory formation in an autoshaping learning task". Pharmacology, Biochemistry, and Behavior. 81 (3): 673–82. doi:10.1016/j.pbb.2005.05.005. PMID 15964617. S2CID 19789219.
| Simple piperazines (no additional rings) |
|
|---|---|
| Phenylpiperazines |
|
| Benzylpiperazines | |
| Diphenylalkylpiperazines (benzhydrylalkylpiperazines) |
|
| Pyrimidinylpiperazines |
|
| Pyridinylpiperazines |
|
| Benzo(iso)thiazolylpiperazines | |
| Tricyclics (piperazine attached via side chain) |
|
| Others/Uncategorized |
|
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.