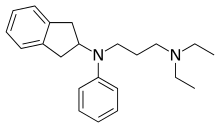
Aprindine
Aprindine is a Class 1b antiarrhythmic agent.[1]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H30N2 |
| Molar mass | 322.496 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
It has been since discovered that the quat salts of Aprindine are also claimed to have an antiarrhythmic activity.[2][3]
Synthesis
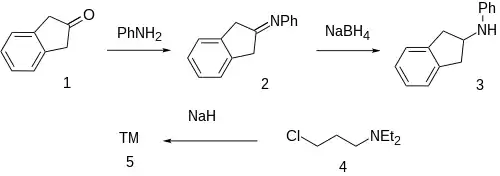
The modern method uses Indene as the starting material since this is more stable than 2-indanone.
The condensation between 2-Indanone [615-13-4] (1) and aniline gives an "anil" type Schiff's base[6] (2). The reduction with sodium borohydride forms the corresponding aminoindane [33237-72-8] (3). Sodium hydride catalyzed alkylation with 3-diethylaminopropyl chloride [104-77-8] (4) completed the synthesis of Aprindine (5).
See also
- Moxaprindine [53076-26-9]
- Pyrophendane [7009-69-0]
- Indriline Fb: [7395-90-6].
References
- WHOCC. "WHOCC - ATC/DDD Index". www.whocc.no. Retrieved 2018-03-01.
- US 3917679, Molloy BB, Tuttle RR, issued 1975, assigned to Eli Lilly and Co
- US 4018897, Molloy BB, issued 1977, assigned to to Eli Lilly and Co
- US 3923813, Vanhoof PM, Clarebout PM, issued 1975, assigned to Christiaens Sa A.
- JPH 1045687, Sasaki T, et al., issued 1998, assigned to Nippon Steel Corp
- "N-(Indan-2-ylidene)aniline". PubChem. U.S. Library of Medicine.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.