Evenamide
Evenamide (INN) (developmental code names NW-3509, NW-3509A) is a selective voltage-gated sodium channel blocker, including (and not limited to) subtypes Nav1.3, Nav1.7, and Nav1.8, which is described as an antipsychotic and is under development by Newron Pharmaceuticals as an add-on therapy for the treatment of schizophrenia.[1][2][3][4] The drug has shown efficacy in animal models of psychosis, mania, depression, and aggression.[3] It has completed phase I clinical trials, and phase II clinical trials will be commenced in the third quarter of 2015.[5]
 | |
| Clinical data | |
|---|---|
| Other names | NW-3509; NW-3509A |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
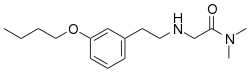
| Formula | C16H26N2O2 |
| Molar mass | 278.396 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
References
- "Drug Development in Schizophrenia: Summary and Table". Pharmaceutical Medicine. 28 (5): 265–271. 2014. doi:10.1007/s40290-014-0070-6. ISSN 1178-2595. S2CID 8513976.
- Progress in Medicinal Chemistry. Elsevier. 6 October 2010. pp. 81–. ISBN 978-0-12-381293-3.
- Gupta SP (21 June 2011). Ion Channels and Their Inhibitors. Springer Science & Business Media. pp. 102–. ISBN 978-3-642-19922-6.
- Zuliani V, Amori L, Rivara M (2011). "Advances in Design and Development of Sodium Channel Blockers". Ion Channels and Their Inhibitors. pp. 79–115. doi:10.1007/978-3-642-19922-6_4. ISBN 978-3-642-19921-9.
- "Newron raised new funds to continue the development of Neurological therapies". Labiotech. 5 February 2015. Archived from the original on 4 March 2016.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.