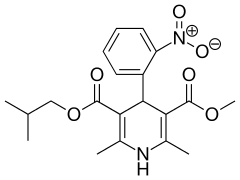
Nisoldipine
Nisoldipine is a pharmaceutical drug used for the treatment of chronic angina pectoris and hypertension. It is a calcium channel blocker of the dihydropyridine class. It is sold in the United States under the proprietary name Sular. Nisoldipine has tropism for cardiac blood vessels.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Sular, Baymycard, Syscor |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696009 |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 4–8% |
| Protein binding | >99% |
| Metabolism | CYP3A4 |
| Elimination half-life | 7–12 hours |
| Excretion | 70–80% via urine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.058.534 |
| Chemical and physical data | |
| Formula | C20H24N2O6 |
| Molar mass | 388.414 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
It was patented in 1975 and approved for medical use in 1990.[2]
Contraindications
Nisoldipine is contraindicated in people with cardiogenic shock, unstable angina, myocardial infarction, and during pregnancy and lactation.[3]
Adverse effects
Common side effects are headache, confusion, fast heartbeat, and edema. Hypersensitivity reactions are rare and include angioedema.[3]
Interactions
The substance is metabolized by the liver enzyme CYP3A4. Consequently, CYP3A4 inducers such as rifampicin or carbamazepine could reduce the effectiveness of nisoldipine, while CYP3A4 inhibitors such as ketoconazole increase the amount of nisoldipine in the body more than 20-fold. Grapefruit juice also increases nisoldipine concentrations by inhibiting CYP3A4.[3]
Pharmacology
Mechanism of action
Nisoldipine is a calcium channel blocker that selectively inhibits L-type calcium channels.[3]
References
- Knorr, Andreas M. (1995). "Why is nisoldipine a specific agent in ischemic left ventricular dysfunction?". The American Journal of Cardiology. 75 (13): E36–E40. doi:10.1016/S0002-9149(99)80446-9. PMID 7726122.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 464. ISBN 9783527607495.
- Haberfeld, H, ed. (2019). Austria-Codex (in German). Vienna: Österreichischer Apothekerverlag. Syscor 5 mg-Filmtabletten.
External links
- Diseases Database (DDB): 30009
- Mielcarek J, Grobelny P, Szamburska O (2005). "The effect of beta-carotene on the photostability of nisoldipine". Methods Find Exp Clin Pharmacol. 27 (3): 167–71. doi:10.1358/mf.2005.27.3.890873. PMID 15834448.
- Missan S, Zhabyeyev P, Dyachok O, Jones SE, McDonald TF (November 2003). "Block of cardiac delayed-rectifier and inward-rectifier K+ currents by nisoldipine". Br. J. Pharmacol. 140 (5): 863–70. doi:10.1038/sj.bjp.0705518. PMC 1574108. PMID 14530219.
- Hamilton S, Houle L, Thadani U (1999). "Rapid-release and coat-core formulations of nisoldipine in treatment of hypertension, angina, and heart failure". Heart Dis. 1 (5): 279–88. PMID 11720635.