Iferanserin
Iferanserin (INN; VEN-309) is a drug which acts as a selective 5-HT2A receptor antagonist.[1] It is under development as an intra-rectal formulation for the treatment of hemorrhoid disease, and as of February 2012, is in phase IIb clinical trials.[1]
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C23H28N2O |
| Molar mass | 348.490 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Synthesis
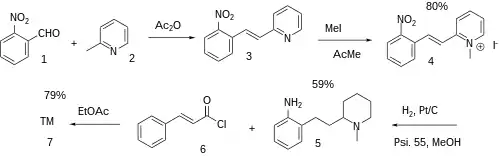
Patent:[2]
The condensation between 2-nitrobenzaldehyde [552-89-6] (1) and 2-picoline [109-06-8] (2) gives 2-(o-nitrostyryl)pyridine [50385-24-5] (3). Alkylation with methyliodide gives the quat salt, CID:54050692 (4). Catalytic hydrogenation at elevated pressure gives 2-(2-aminophenethyl)-1-methylpiperidine, CID:11085282 (5). Separation of the enantiomers and amide formation with cinnamoyl chloride [17082-09-6] (6) completed the synthesis of Iferanserin (7).
See also
- Encainide
- Modecainide [81329-71-7]
References
- Herold A, Dietrich J, Aitchison R (February 2012). "Intra-anal Iferanserin 10 mg BID for hemorrhoid disease: a prospective, randomized, double-blind, placebo-controlled trial". Clinical Therapeutics. 34 (2): 329–40. doi:10.1016/j.clinthera.2011.12.012. PMID 22244049.
- Moh Samir Amer, WO 2012026914 (2012).
Drugs for functional gastrointestinal disorders (A03) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drugs for functional bowel disorders |
| ||||||||||||
| Belladonna and derivatives (antimuscarinics) |
| ||||||||||||
| Propulsives | |||||||||||||
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.