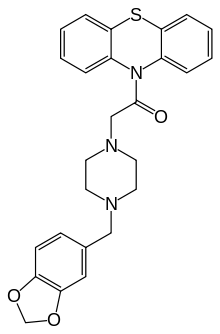
Fenoverine
Fenoverine (INN) is an antispasmodic [also known as spasmolytics] drug,[1] which acts by inhibiting calcium channels[2] [much in the same way as traditional calcium channel blockers, which are used as antianginal drugs]. In the case of Fenoverine, the relaxation occurs in abdominal / intestinal smooth mucles, while in case of antianginal drugs, the relaxation occurs in coronary vessels. Notably Fenoverine does not act as an antianginal agent.
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.666 |
| Chemical and physical data | |
| Formula | C26H15N3O3S |
| Molar mass | 449.48 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Toxicity
Fenoverine is known to cause rhabdomyolysis.[2][3]
Synthesis
Note that the synthesis involves MDBZP agent.
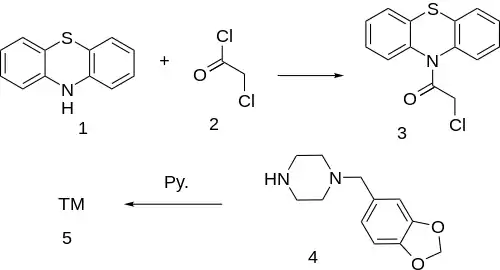
Acylation of phenothiazine (1) with chloroacetyl chloride [79-04-9] (2) gives 10-(Chloroacetyl)-Phenothiazine [786-50-5] (3). Displacement of the remaining halogen upon addition of 1-Piperonylpiperazine [32231-06-4] (4) in pyridine completes the synthesis of Fenoverine (5).
References
- Martínez-Vázquez MA, Vázquez-Elizondo G, González-González JA, Gutiérrez-Udave R, Maldonado-Garza HJ, Bosques-Padilla FJ (2012). "Effect of antispasmodic agents, alone or in combination, in the treatment of Irritable Bowel Syndrome: systematic review and meta-analysis". Revista De Gastroenterologia De Mexico. 77 (2): 82–90. doi:10.1016/j.rgmx.2012.04.002. PMID 22672854.
- Chariot P, Ratiney R, Le Maguet F, Fourestié V, Astier A, Gherardi R (August 1995). "Fenoverine-induced rhabdomyolysis". Hum Exp Toxicol. 14 (8): 654–656. doi:10.1177/096032719501400805. PMID 7576832.
- Cho J, Na J, Bae E, Lee TW, Jang HN, Cho HS, Chang SH, Park DJ (April 2020). "The incidence, risk factors, and clinical outcomes of rhabdomyolysis associated with fenoverine prescription: a retrospective study in South Korea (1999-2014)". BMC Pharmacol Toxicol. 21 (1): 30. doi:10.1186/s40360-020-00408-3. PMC 7183697. PMID 32334639.
- Buzas Andre & Pierre Rene, FR2092639 (1972).