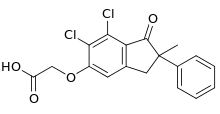
Indacrinone
Indacrinone is a loop diuretic. It can be used in patients of gout with hypertension as an antihypertensive because it decreases reabsorption of uric acid,[1] while other diuretics increase it.
 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ECHA InfoCard | 100.054.496 |
| Chemical and physical data | |
| Formula | C18H14Cl2O4 |
| Molar mass | 365.21 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Chirality and biological activity
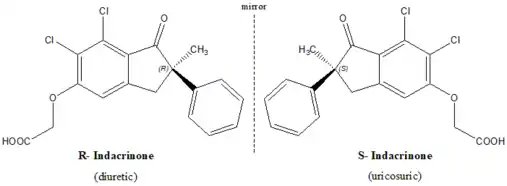
Indacrinone is a chiral drug, with one chiral center and hence exists as mirror-image twins. (R)-enantiomer, the eutomer, is diuretic whereas the mirror-image version (S)-enantiomer counteracts side effect of the eutomer. Here both the enantiomers contribute to the overall desired effect in different ways.
As indicated earlier, the (R)- enantiomer is the pharmacologically active diuretic. Like most other diuretics, the (R)-isomer possesses an undesirable side-effect of retaining uric acid. But the (S)-enantiomer, the distomer, has the property of assisting uric acid secretion (uricosuric effect), and, therefore, antagonizing the undesirable side-effects of the eutomer (uric-acid retention).[2][3] It affords a good argument for the marketing of a racemic mixture. But studies exemplify that 9:1 mixture of the two enantiomers provides optimal therapeutic value.[4]
See also
- Chiral drugs
- Chirality
- Eudisimic ratio
References
- Vlasses PH, Rotmensch HH, Swanson BN, Irvin JD, Johnson CL, Ferguson RK (1984). "Indacrinone: natriuretic and uricosuric effects of various ratios of its enantiomers in healthy men". Pharmacotherapy. 4 (5): 272–7. doi:10.1002/j.1875-9114.1984.tb03374.x. PMID 6504708. S2CID 19743065.
- Ariëns, Everardus J. (1986). "Stereochemistry: A source of problems in medicinal chemistry". Medicinal Research Reviews. 6 (4): 451–466. doi:10.1002/med.2610060404. ISSN 0198-6325.
- Kannappan, Valliappan. "Indacrinone – Chiralpedia". Retrieved 2022-08-28.
- The impact of stereochemistry on drug development and use. Hassan Y. Aboul-Enein, Irving W. Wainer. New York: Wiley. 1997. ISBN 0-471-59644-2. OCLC 35262289.
{{cite book}}: CS1 maint: others (link)