Netarsudil
Netarsudil, sold under the brand name Rhopressa among others, is a medication for the treatment of glaucoma.[1][2][3] In the United States, in December 2017, the Food and Drug Administration (FDA) approved a 0.02% ophthalmic solution for the lowering of elevated intraocular pressure in people with open-angle glaucoma or ocular hypertension.[4][5] The European Medicines Agency approved it in 2019 for the same uses under the brand name Rhokiinsa.[2]
 | |
| Clinical data | |
|---|---|
| Pronunciation | ne TAR soo dil |
| Trade names | Rhopressa, Rhokiinsa |
| Other names | AR-13324 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618014 |
| License data |
|
| Routes of administration | Eye drops, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Metabolism | Esterases in the cornea |
| Metabolites | AR-13503 (active metabolite) |
| Elimination half-life | 16–17 hrs |
| Duration of action | ≥ 24 hrs |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ECHA InfoCard | 100.251.524 |
| Chemical and physical data | |
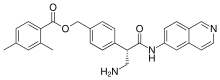
| Formula | C28H27N3O3 |
| Molar mass | 453.542 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
The FDA considers it to be a first-in-class medication.[6]
Contraindications
Netarsudil has no contraindications apart from known hypersensitivity to the drug.[2][7]
Adverse effects
The most common side effects are hyperaemia (increased blood flow associated with redness, in 51% of patients) in the conjunctiva, cornea verticillata (drug deposits in the cornea, in 17%), and eye pain (in 17%). All other side effects occur in fewer than 10% of people. Hypersensitivity reactions occur in fewer than 1%.[2][8]
Overdose
Overdosing netarsudil is unlikely because concentrations in the body are so low that they are generally not detectable.[8]
Interactions
No interaction studies have been done. The European label recommends to apply other eye drops at least five minutes before, and eye ointments at least five minutes after netarsudil drops.[2][7]
Pharmacology
Mechanism of action
This drug's mechanism of action is not entirely clear. It inhibits the enzyme rho kinase. This appears to increase outflow of aqueous humor through the trabecular meshwork, and also to reduce pressure in the veins of the episcleral layer. The drug also inhibits the norepinephrine transporter.[2][7]
Pharmacokinetics
After instillation into the eye, netarsudil is cleaved by esterases in the cornea to AR-13503, which is the active metabolite. Concentrations reached in the blood plasma are so low that they are generally not detectable. To judge from animal models, the drug acts for at least 24 hours. Its elimination half-life is 16 to 17 hours (in rabbits).[7]
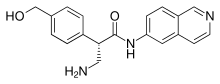
Chemistry
The drug is used in form of a salt, netarsudil dimesilate, which is a white to light yellow crystalline powder. It is a weak acid and moderately hygroscopic, freely soluble in water and soluble in methanol.[9]
See also
- Netarsudil/latanoprost, a combination drug
References
- "Rhopressa- netarsudil solution/ drops". DailyMed. Retrieved 2 May 2021.
- "Rhokiinsa EPAR". European Medicines Agency (EMA). 16 September 2019. Retrieved 27 September 2020.
- Dasso L, Al-Khaled T, Sonty S, Aref AA (2018). "Profile of netarsudil ophthalmic solution and its potential in the treatment of open-angle glaucoma: evidence to date". Clinical Ophthalmology. Auckland, N.Z. 12: 1939–1944. doi:10.2147/OPTH.S154001. PMC 6177382. PMID 30323550.
- "Rhopressa (netarsudil) Ophthalmic Solution". U.S. Food and Drug Administration (FDA). 29 January 2018. Retrieved 3 June 2020.
- "Aerie (AERI) Gets Early FDA Approval for Lead Drug Rhopressa". 19 December 2017.
- New Drug Therapy Approvals 2017 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2018. Retrieved 16 September 2020.
- "Netarsudil Mesylate Monograph for Professionals". American Society of Health-System Pharmacists. 16 November 2020. Retrieved 2 May 2021.
- "Rhokiinsa Product Information" (PDF). European Medicines Agency (EMA). 2 December 2019.
- "Rhokiinsa: EPAR – Public assessment report" (PDF). European Medicines Agency. 3 December 2019.
External links
- "Netarsudil". Drug Information Portal. U.S. National Library of Medicine.
- "Netarsudil mesylate". Drug Information Portal. U.S. National Library of Medicine.