Omidenepag
Omidenepag, sold under the brand name Eybelis among others, is a medication used for the treatment of glaucoma and ocular hypertension.[1][2]
 | |
| Clinical data | |
|---|---|
| Trade names | Eybelis, Omlonti |
| Other names | UR-7276, DE-117, Omidenepag isopropyl (JAN JP) |
| Routes of administration | Topical eye drops |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
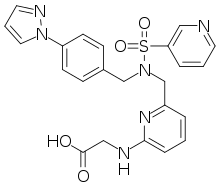
| Formula | C26H28N6O4S |
| Molar mass | 520.61 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Omidenepag was approved for medical use in Japan in 2018,[2] and in the United States in September 2022.[3][4]
Adverse effects
The most common adverse effects of omidenepag are conjunctival hyperemia and macular edema, including cystoid macular edema.[2]
Pharmacology
Omidenepag isopropyl is a prodrug that is converted by hydrolysis of its isopropyl ester to the active metabolite omidenepag.[5] Omidenepag is a selective prostaglandin E2 receptor agonist.[6][7]
History
Omidenepag was developed by Ube Industries and Santen Pharmaceutical.[2]
References
- "Omlonti- omidenepag isopropyl solution/ drops". DailyMed. 30 September 2022. Retrieved 16 October 2022.
- Duggan S (December 2018). "Omidenepag Isopropyl Ophthalmic Solution 0.002%: First Global Approval". Drugs. 78 (18): 1925–1929. doi:10.1007/s40265-018-1016-1. PMID 30465134. S2CID 53721056.
- "Omlonti: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 23 September 2022. Retrieved 23 September 2022.
- "Santen and UBE Received FDA Approval for Omlonti (Omidenepag Isopropyl Ophthalmic Solution) 0.002% for the Reduction of Elevated Intraocular Pressure in Patients with Primary Open-Angle Glaucoma or Ocular Hypertension" (Press release). Santen. 26 September 2022. Retrieved 1 October 2022 – via Business Wire.
- "Omidenepag isopropyl". DrugCentral. Division of Translational Informatics at University of New Mexico. Archived from the original on 8 January 2022. Retrieved 8 January 2022.
- Kirihara T, Taniguchi T, Yamamura K, Iwamura R, Yoneda K, Odani-Kawabata N, et al. (January 2018). "Pharmacologic Characterization of Omidenepag Isopropyl, a Novel Selective EP2 Receptor Agonist, as an Ocular Hypotensive Agent". Investigative Ophthalmology & Visual Science. 59 (1): 145–153. doi:10.1167/iovs.17-22745. PMID 29332128.
- Ida Y, Hikage F, Umetsu A, Ida H, Ohguro H (September 2020). "Omidenepag, a non-prostanoid EP2 receptor agonist, induces enlargement of the 3D organoid of 3T3-L1 cells". Scientific Reports. 10 (1): 16018. Bibcode:2020NatSR..1016018I. doi:10.1038/s41598-020-72538-x. PMC 7524797. PMID 32994409.
External links
- "Omidenepag". Drug Information Portal. U.S. National Library of Medicine.
- "Omidenepag isopropyl". Drug Information Portal. U.S. National Library of Medicine.
- "Omidenepag". NCI Thesaurus.
- "Omidenepag isopropyl". NCI Thesaurus.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.