Vericiguat
Vericiguat, sold under the brand name Verquvo, is a medication used to reduce the risk of cardiovascular death and hospitalization in certain patients with heart failure after a recent acute decompensation event.[3][4][5] It is taken by mouth.[3][4][5]
 | |
| Clinical data | |
|---|---|
| Trade names | Verquvo |
| License data |
|
| Pregnancy category | |
| Routes of administration | By mouth |
| Drug class | Soluble guanylate cyclase activator |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.247.370 |
| Chemical and physical data | |
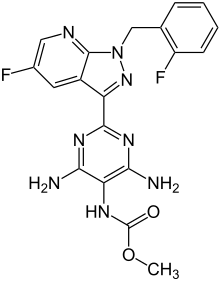
| Formula | C19H16F2N8O2 |
| Molar mass | 426.388 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Common side effects include low blood pressure and low red cell count (anemia).[4][5]
Vericiguat is a soluble guanylate cyclase (sGC) stimulator.[3] It was approved for medical use in the United States in January 2021,[4][6] and for use in the European Union in July 2021.[5]
Medical uses
Vericiguat is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure following a prior hospitalization for heart failure or need for outpatient intravenous diuretics, in adults with symptomatic chronic heart failure and an ejection fraction of less than 45%.[3][4]
Adverse effects
Vericiguat causes harm to the unborn baby and should not be given to pregnant women.[4] It is also not known how vericiguat passes into breastmilk, therefore patients should not take vericiguat The most common side effects of vericiguat include low blood pressure and anemia.[3] Patients taking other soluble guanylate cyclase inhibitors should also not be taking vericiguat.[3]
Pharmacology
Vericiguat is a direct stimulator of soluble guanylate cyclase enzyme, an important enzyme in vascular smooth muscle cells. Specifically, vericiguat will bind to the beta-subunit of the target site on the soluble guanylate cyclase enzyme.[7] Soluble guanylate cyclase enzymes catalyzes the formation of cyclic GMP upon interaction with nitric oxide to activate a number of downstream signaling cascade which can compensate for defects in this pathway and resulting losses in regulatory myocardial and vascular cellular processes due to cardiovascular complications. [7]
Pharmacokinetics
After vericiguat is administered (100 mg by mouth once daily), the average steady state and Cmax and AUC for patients with cardiovascular failure is 350 mcg/L and 6,680 mcg/h/L with a Tmax of one hour. Vericiguat has a positive food effect, and therefore patients are advised to consume food with the drug for an oral bioavailability of 93%. [7] Vericiguat is extensively protein-bound in plasma . [7] Vericiguat is primarily metabolized via phase II conjugation reactions, with a minor CYP-mediated oxidative metabolite. The major metabolite is glucuronidated and inactive. The typical half-life profile for patients with heart failure is 30 hours. Vericiguat has a decreased clearance and was observed to have a 1.6 g/L clearance in patients with systolic heart failure. [7]
History
The U.S. Food and Drug Administration (FDA) approved vericiguat based on evidence from a clinical trial (NCT02861534) which consisted of 5,050 participants aged 23 to 98 years old with worsening heart failure.[4] The trial was conducted at 694 sites in 42 countries in Europe, Asia, North and South America.[4] The trial enrolled participants with symptoms of worsening heart failure.[4] Participants were randomly assigned to receive vericiguat or a placebo pill once a day.[4] Neither the participants nor the health care professionals knew if the participants were given vericiguat or placebo pill until after the trial was complete.[4] It was awarded a fast track designation on January 19th, 2021. [8]
Society and culture
Legal status
On 20 May 2021, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for vericiguat, intended for the treatment of symptomatic chronic heart failure in adults with reduced ejection fraction.[9] The applicant for this medicinal product is Bayer AG. Vericiguat was approved for medical use in the European Union in July 2021.[5]
References
- "Verquvo". Therapeutic Goods Administration (TGA). 29 November 2021. Retrieved 28 December 2021.
- "Updates to the Prescribing Medicines in Pregnancy database". Therapeutic Goods Administration (TGA). 12 May 2022. Retrieved 13 May 2022.
- "Verquvo- vericiguat tablet, film coated". DailyMed. Retrieved 9 February 2021.
- "Drug Trials Snapshot: Verquvo". U.S. Food and Drug Administration (FDA). 8 February 2021. Retrieved 8 February 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Verquvo EPAR". European Medicines Agency (EMA). 19 May 2021. Retrieved 14 September 2021.
- "Drug Approval Package: Verquvo". U.S. Food and Drug Administration (FDA). 17 February 2021. Retrieved 14 September 2021.
- "Vericiguat". go.drugbank.com. Retrieved 9 April 2022.
- Hochron, By Adam. "FDA Grants Fast Track Designation for Heart Failure Developmental Treatment". www.mdalert.com. Retrieved 9 April 2022.
- "Verquvo: Pending EC decision". European Medicines Agency. 20 May 2021. Retrieved 23 May 2021. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
Further reading
- Armstrong PW, Pieske B, Anstrom KJ, Ezekowitz J, Hernandez AF, Butler J, et al. (May 2020). "Vericiguat in Patients with Heart Failure and Reduced Ejection Fraction". N Engl J Med. 382 (20): 1883–1893. doi:10.1056/NEJMoa1915928. PMID 32222134.
External links
- "Vericiguat". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT02861534 for "A Study of Vericiguat in Participants With Heart Failure With Reduced Ejection Fraction (HFrEF) (MK-1242-001) (VICTORIA)" at ClinicalTrials.gov