Amitifadine
Amitifadine (developmental code names DOV-21,947, EB-1010) is a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) or so-called triple reuptake inhibitor (TRI) which is or was being developed by Euthymics Bioscience[1][2] It was under development for the treatment of major depressive disorder, but in May 2013, it was reported that the drug failed to show superior efficacy to placebo in a phase IIb/IIIa clinical trial.[3] It was suggested that this may have been due to the drug being underdosed.[3] In September 2017, development of amitifadine for the treatment of major depressive disorder was finally officially discontinued.[1] As of September 2017, it is still listed as being under development for the treatment of alcoholism and smoking withdrawal.[1]
 | |
| Clinical data | |
|---|---|
| Pronunciation | (/æmɪˈtɪfədiːn/ am-i-TIF-ə-deen) |
| Other names | DOV-21,947; EB-1010 |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
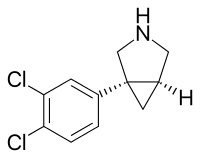
| Formula | C11H11Cl2N |
| Molar mass | 228.12 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Pharmacology
Ki values for SERT, NET, and DAT of amitifadine are 99 nM, 262 nM, and 213 nM.[2] The IC50 values for serotonin, norepinephrine and dopamine uptake are 12, 23 and 96 nM, respectively
| Compound | Uptake (IC50, nM) | Binding (Ki, nM) | ||||
|---|---|---|---|---|---|---|
| 5-HT | NE | DA | SERT | NET | DAT | |
| Amitifadine | 12 | 23 | 96 | 100 | 260 | 210 |
| DOV-216,303 | 14 | 20 | 78 | 190 | 380 | 190 |
| DOV-102,677 | 130 | 100 | 130 | 740 | 1000 | 220 |
Amitifadine reduces the duration of immobility in the forced swim test in rats with an oral minimum effective dose (MED) of 5 mg/kg. This antidepressant-like effect manifests in the absence of significant increases in motor activity at doses of up to 20 mg/kg. Amitifadine also produces a dose-dependent reduction in immobility in the tail suspension test, with an oral MED of 5 mg/kg. In microdialysis studies, amitifadine increased extracellular levels of serotonin, norepinephrine and dopamine in brain regions and did not induce hyperactivity in rats.[4] Results in a small clinical trial indicated that amitifadine had statistically significant antidepressant effects and was well tolerated.[5]
Chemistry

Amitifadine is the (+)-enantiomer of DOV-216,303, and its (−)-enantiomer is DOV-102,677.
Amitifadine is very similar in structure to Bicifadine & Centanafadine.
A so-called "bifunctional molecule" from a separate organization called GSK 598809 has related structure.
References
- "Amitifadine". AdisInsight. Retrieved 26 February 2017.
- Skolnick P, Popik P, Janowsky A, Beer B, Lippa AS (February 2003). "Antidepressant-like actions of DOV 21,947: a "triple" reuptake inhibitor". European Journal of Pharmacology. 461 (2–3): 99–104. doi:10.1016/S0014-2999(03)01310-4. PMID 12586204.
- "Euthymics Reports Top-Line Results From Triade Trial of Amitifadine for Major Depressive Disorder" (PDF). Cambridge, MA: Euthymics Bioscience, Inc. May 29, 2013. Archived from the original (PDF) on 2017-09-24. Retrieved 2017-06-12.
- Golembiowska K, Kowalska M, Bymaster FP (May 2012). "Effects of the triple reuptake inhibitor amitifadine on extracellular levels of monoamines in rat brain regions and on locomotor activity". Synapse. 66 (5): 435–44. doi:10.1002/syn.21531. PMID 22213370. S2CID 32581051.
- Tran P, Skolnick P, Czobor P, Huang NY, Bradshaw M, McKinney A, Fava M (January 2012). "Efficacy and tolerability of the novel triple reuptake inhibitor amitifadine in the treatment of patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial". Journal of Psychiatric Research. 46 (1): 64–71. doi:10.1016/j.jpsychires.2011.09.003. PMID 21925682.