Talopram
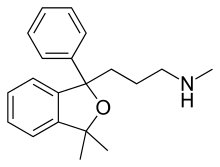
Talopram (Lu 3-010),[1] also known as phthalapromine, is a selective norepinephrine reuptake inhibitor (NRI) which was researched for the management of depression in the 1960s and 1970s but was never commercialized. Along with talsupram, talopram is structurally related to the selective serotonin reuptake inhibitor (SSRI) citalopram,[2] as well as to melitracen:[3]
In 1971, the company hired Klaus Bøgesø as a medicinal chemist. Over the years Bøgesø turned out to have a Midas touch at the game of drug hunting, creating more molecules that made it to the market than almost any other medicinal chemist in the field. The challenge facing him in 1971 following his recruitment was to produce a selective norepinephrine reuptake inhibitor. Like other companies at the time, Lundbeck had little interest in an SSRI. Bøgesø began from an accident in the laboratory. Trying to create a derivative of their norepinephrine reuptake inhibiting antidepressant melitracen, Lundbeck chemists accidentally produced a new chemical — a phenylphthalene. Against all the odds, just like melitracen, this was also a selective norepinephrine reuptake inhibitor. Two potential antidepressants came out of this — talopram and tasulopram, which were pressed into clinical trials. Both however turned out to be energizing, and in a number of cases there were suicide attempts. The fact that there were suicide attempts appeared to confirm another proposal of Paul Kielholz, that activating antidepressants might lead to suicide. Lundbeck's experience suggested that norepinephrine reuptake inhibitors were likely to lead to just this problem. Lundbeck retreated, scared. If norepinephrine reuptake inhibitors were likely to trigger suicide, the greatest hazard of an antidepressant, then Kielholz's view suggested that an SSRI would be less likely to lead to suicide. Bøgesø's job was to see whether the new series of drugs could be converted into a series of SSRIs. Following a lead from Carlsson on how to do this, he converted talopram into citalopram, the most selective serotonin reuptake inhibitor to come to the market.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C20H25NO |
| Molar mass | 295.426 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
Synthesis
An unexpected/fortuitous rearrangement product in the synthesis of litracen is what led to talopram.[3]
The Grignard reaction between 3,3-dimethylphthalide [1689-09-4] (1) and phenyl magnesium bromide gives 3,3-dimethyl-1-phenyl-1-phthalanol [1023-91-2]. Dehydration of the tertiary carbinol with perchloric acid [7601-90-3] gives oxonium ion, CID:24213121 (2). Conversion of dimethylaminopropyl chloride [109-54-6] (3) into the Grignard reagent, which further reacts to give CID:114378 (4). Refluxing with ethyl chlorocarbonate gives an intermediate carbamate, which is hydrolysed giving talopram (5).
See also
- Talsupram (tasulopram)
- Amedalin
- Daledalin
- U.S. Patent 3,467,675, 1969 (#15).
- Original literature[8]
- Prindamine (21489-22-5)[9]
References
- Carlsson A, Fuxe K, Hamberger B, Malmfors T (May 1969). "Effect of a new series of bicyclic compounds with potential thymoleptic properties on the reserpine-resistant uptake mechanism of central and peripheral monoamine neurones in vivo and in vitro". British Journal of Pharmacology. 36 (1): 18–28. doi:10.1111/j.1476-5381.1969.tb08299.x. PMC 1703539. PMID 5768100.
- Eildal JN, Andersen J, Kristensen AS, Jørgensen AM, Bang-Andersen B, Jørgensen M, Strømgaard K (May 2008). "From the selective serotonin transporter inhibitor citalopram to the selective norepinephrine transporter inhibitor talopram: synthesis and structure-activity relationship studies". Journal of Medicinal Chemistry. 51 (10): 3045–8. doi:10.1021/jm701602g. PMID 18429609.
- Healy D. "The History of the SSRIs" (PDF). Let Them Eat Prozac.
- Wang, C. H., Isensee, R., Griffith, A. M., Christensen, B. E. (August 1947). "The Preparation of 3,3-Dimethylphthalide and Several of its Derivatives 1". Journal of the American Chemical Society. 69 (8): 1909–1911. doi:10.1021/ja01200a019. ISSN 1520-5126 0002-7863, 1520-5126.
{{cite journal}}: Check|issn=value (help) - Povl Viggo Petersen, et al. US3467675 (1969 to KEFALAS AS). (#15).
- Anon, NL6603606 (1966); CA, 66, 65392m (synth, pharmacol)
- Povl Viggo Peterson, et al. GB1102762 (1968 to KEFALAS AS).
- Petersen PV, Lassen N, Hansen V, Huld T, Hjortkjaer J, Holmblad J, et al. (2009). "Pharmacological studies of a new series of bicyclic thymoleptics". Acta Pharmacologica et Toxicologica. 24 (2): 121–33. doi:10.1111/j.1600-0773.1966.tb00375.x. PMID 4165059.
- "Prindamine". ChemicalBook.
