Dimethylmercury
Dimethylmercury ((CH3)2Hg) is an extremely toxic organomercury compound. A highly volatile, reactive, flammable, and colorless liquid, dimethylmercury is one of the strongest known neurotoxins, with a quantity of less than 0.1 mL capable of inducing severe mercury poisoning resulting in death, and is easily absorbed through the skin. Dimethylmercury is capable of permeating many materials, including plastic and rubber compounds. It has a slightly sweet odor.[2]
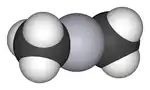 | |
| Names | |
|---|---|
| IUPAC name
Dimethylmercury[1] | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
Beilstein Reference |
3600205 |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.916 |
| EC Number |
|
Gmelin Reference |
25889 |
| MeSH | dimethyl+mercury |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2929 |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C 2H 6Hg (CH 3) 2Hg |
| Molar mass | 230.66 g mol−1 |
| Appearance | Colorless liquid |
| Odor | Sweet |
| Density | 2.961 g mL−1 |
| Melting point | −43 °C (−45 °F; 230 K) |
| Boiling point | 93 to 94 °C (199 to 201 °F; 366 to 367 K) |
Refractive index (nD) |
1.543 |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
57.9–65.7 kJ mol−1 |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Extremely flammable, extremely poisonous, persistent environmental pollutant |
| GHS labelling: | |
Pictograms |
    |
Signal word |
Danger |
Hazard statements |
H224, H300+H310+H330, H372, H410 |
Precautionary statements |
P260, P264, P273, P280, P284, P301+P310 |
| NFPA 704 (fire diamond) | |
| Flash point | 5 °C (41 °F; 278 K) |
| Related compounds | |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis, structure, and reactions
The compound was one of the earliest organometallics reported, reflecting its considerable stability. The compound was first prepared by George Buckton in 1857 by a reaction of methylmercury iodide with potassium cyanide:[3]
- 2 CH3HgI + 2 KCN → Hg(CH3)2 + 2 KI + (CN)2 + Hg
Later, Frankland discovered that it could be synthesized by treating sodium amalgam with methyl halides:
- Hg + 2 Na + 2 CH3I → Hg(CH3)2 + 2 NaI
It can also be obtained by alkylation of mercuric chloride with methyllithium:
- HgCl2 + 2 LiCH3 → Hg(CH3)2 + 2 LiCl
The molecule adopts a linear structure with Hg–C bond lengths of 2.083 Å.[4]
Reactivity and physical properties
An unusual feature of this compound is its low reactivity towards proton sources, being stable in water and reacting with mineral acids at a significant rate only at elevated temperatures,[5][6] whereas the corresponding organocadmium and organozinc compounds (and most metal alkyls in general) hydrolyze rapidly. The difference reflects the high electronegativity of Hg (Pauling EN = 2.00) low affinity of Hg(II) for oxygen ligands. The compound undergoes a redistribution reaction with mercuric chloride to give methylmercury chloride:
- (CH3)2Hg + HgCl2 → 2 CH3HgCl
Whereas dimethylmercury is a volatile liquid, methylmercury chloride is a crystalline solid.[7]
Use
Dimethylmercury currently has few applications because of the risks involved. As with many methyl-organometallics, it is a methylating agent that can donate its methyl groups to an organic molecule; however, the development of less toxic nucleophiles such as dimethylzinc, trimethylaluminium, and Grignard reagents (organomagnesium halides), has essentially rendered this compound obsolete in organic chemistry. It was also studied for reactions involving bonding methylmercury cations to target molecules, forming potent bactericides, but methylmercury's bioaccumulation and ultimate toxicity has led it to be largely abandoned in favor of the less toxic ethylmercury and diethylmercury compounds, which perform a similar function without the bioaccumulation hazard.
In toxicology, it still finds limited use as a reference toxin. It is also used to calibrate NMR instruments for detection of mercury (δ 0 ppm for 199Hg NMR), although diethylmercury and less toxic mercury salts are now preferred.[8][9][10]
Safety
Dimethylmercury is extremely toxic and dangerous to handle. Absorption of doses as low as 0.1 mL can result in severe mercury poisoning.[2] The risks are enhanced because of the compound's high vapor pressure.[2] Medicinal chemist Derek Lowe called it "deadly and hideous" in a 2013 article.[11]
Permeation tests showed that several types of disposable latex or polyvinyl chloride gloves (typically, about 0.1 mm thick), commonly used in most laboratories and clinical settings, had high and maximal rates of permeation by dimethylmercury within 15 seconds.[12] The American Occupational Safety and Health Administration advises handling dimethylmercury with highly resistant laminated gloves with an additional pair of abrasion-resistant gloves worn over the laminate pair, and also recommends using a face shield and working in a fume hood.[2][13]
Dimethylmercury is metabolized after several days to methylmercury.[12] Methylmercury crosses the blood–brain barrier easily, probably owing to formation of a complex with cysteine.[13] It is not quickly eliminated from the organism, and therefore has a tendency to bioaccumulate. The symptoms of poisoning may be delayed by months, resulting in cases in which a diagnosis is ultimately discovered, but only at a point in which it is too late or almost too late for an effective treatment regimen to be successful.[13] Methylmercury poisoning is also known as Minamata disease.
Incidents
As early as 1865, two workers in the laboratory of Frankland died after exhibiting progressive neurological symptoms following accidental exposure to the compound.[3]
Karen Wetterhahn, a professor of chemistry at Dartmouth College, died in 1997, ten months after spilling only a few drops of dimethylmercury onto her latex gloves.[2][14] This incident resulted in improved safety procedures for chemical-protection clothing and fume hood use.[15]
On 15 July 2011, a German man was stabbed with an umbrella in the city of Hanover, Germany. The man, who died a year later, had managed to take the syringe from the umbrella, which was later analyzed to contain dimethylmercury;[16] the reported cause of death was mercury poisoning.[17][18][19]
See also
- Diethylmercury
- Mercury poisoning
- Minamata disease
- Methylmercury
References
- "dimethylmercury – Compound Summary". PubChem Compound. US: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Retrieved 29 January 2021.
- "OSHA Hazard Information Bulletins – Dimethylmercury". OSHA.gov. Retrieved 29 January 2021.
- The Chemistry of mercury. C. A. McAuliffe. London: Macmillan. 1977. ISBN 978-1-349-02489-6. OCLC 1057702183.
{{cite book}}: CS1 maint: others (link) - Holleman, A. F.; Wiberg, Egon; Wiberg, Nils (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- Crabtree, Robert H. (2005). The Organometallic Chemistry of the Transition Metals (4th ed.). Hoboken, N.J.: John Wiley. p. 424. ISBN 0471662569. OCLC 61520528.
- Baughman, George L.; Gordon, John A.; Wolfe, N. Lee; Zepp, Richard G. (September 1973). Chemistry of Organomercurials in Aquatic Systems. United States Environmental Protection Agency Ecological Research Series. U.S. Govt. Print. Off. pp. 34–40. Retrieved 29 January 2021.
- "Methylmercury chloride". PubChem. National Center for Biotechnology Information, United States National Institutes of Health. Retrieved 29 January 2021.
- O'Halloran, T. V.; Singer, C. P. (10 March 1998). "199Hg Standards". Northwestern University. Archived from the original on 14 May 2005. Retrieved 20 January 2021.
- Hoffman, R. (1 August 2011). "(Hg) Mercury NMR". Jerusalem: The Hebrew University. Retrieved 29 January 2021.
- "Delayed Toxic Syndromes" (PDF). Terrorism by Fear and Uncertainty. ORAU. Archived from the original (PDF) on 23 April 2012. Retrieved 29 January 2021.
- Derek Lowe (8 May 2013). "Things I Won't Work With: Dimethylcadmium". Science. Retrieved 28 June 2022.
- Nierenberg, David W.; Nordgren, Richard E.; Chang, Morris B.; Siegler, Richard W.; Blayney, Michael B.; Hochberg, Fred; Toribara, Taft Y.; Cernichiari, Elsa; Clarkson, Thomas (1998). "Delayed Cerebellar Disease and Death after Accidental Exposure to Dimethylmercury". New England Journal of Medicine. 338 (23): 1672–1676. doi:10.1056/NEJM199806043382305. PMID 9614258.
- Cotton, Simon (October 2003). "Dimethylmercury and Mercury Poisoning: The Karen Wetterhahn story". Molecule of the Month. Bristol University School of Chemistry. doi:10.6084/m9.figshare.5245807. Retrieved 29 January 2021.
- "DimethylMercury and Mercury poisoning". Molecule of the Month www.chm.bris.ac.uk. October 2003. Retrieved 25 August 2022.
- Cavanaugh, Ray (19 February 2019). "The dangers of dimethylmercury". Chemistry World. Royal Society of Chemistry. Retrieved 29 January 2021.
- "Namen genannt! Wird der Regenschirm-Mord an Familienvater Christoph (†40) endlich gelöst?". TAG24 (in German). 25 August 2022. Archived from the original on 25 August 2022. Retrieved 30 August 2022.
- Albers, Anne; Gies, Ursula; Raatschen, Hans-Jurgen; Klintschar, Michael (1 September 2020). "Another umbrella murder? – A rare case of Minamata disease". Forensic Science, Medicine and Pathology. 16 (3): 504–509. doi:10.1007/s12024-020-00247-y. ISSN 1556-2891. PMC 7449996. PMID 32323188.
- "Umbrella stab victim dies of mercury poisoning". www.thelocal.de. 11 May 2012. Retrieved 13 June 2022.
- "Quecksilbervergiftung" [Mercury poisoning]. Der Spiegel (in German). 11 May 2012. Retrieved 3 September 2020.
