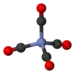
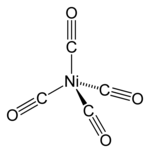
Nickel tetracarbonyl
Nickel carbonyl (IUPAC name: tetracarbonylnickel) is a nickel(0) organometallic compound with the formula Ni(CO)4. This colorless liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for producing very high-purity nickel and a reagent in organometallic chemistry, although the Mond Process has fallen out of common usage due to the health hazards in working with the compound. Nickel carbonyl is one of the most dangerous substances yet encountered in nickel chemistry due to its very high toxicity, compounded with high volatility and rapid skin absorption.[4]
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Tetracarbonylnickel | |||
| Other names
Nickel tetracarbonyl Nickel carbonyl (1:4) | |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
Beilstein Reference |
6122797 | ||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.033.322 | ||
| EC Number |
| ||
Gmelin Reference |
3135 | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1259 | ||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
Ni(CO)4 | ||
| Molar mass | 170.73 g/mol | ||
| Appearance | colorless liquid[1] | ||
| Odor | musty,[1] like brick dust | ||
| Density | 1.319 g/cm3 | ||
| Melting point | −17.2 °C (1.0 °F; 256.0 K) | ||
| Boiling point | 43 °C (109 °F; 316 K) | ||
Solubility in water |
0.018 g/100 mL (10 °C) | ||
| Solubility | miscible in most organic solvents soluble in nitric acid, aqua regia | ||
| Vapor pressure | 315 mmHg (20 °C)[1] | ||
| Viscosity | 3.05 x 10−4 Pa s | ||
| Structure | |||
Coordination geometry |
Tetrahedral | ||
Molecular shape |
Tetrahedral | ||
Dipole moment |
zero | ||
| Thermochemistry | |||
Std molar entropy (S⦵298) |
320 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
−632 kJ/mol | ||
Std enthalpy of combustion (ΔcH⦵298) |
−1180 kJ/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Potential occupational carcinogen[2] | ||
| GHS labelling: | |||
Pictograms |
    | ||
Hazard statements |
H225, H300, H301, H304, H310, H330, H351, H360D, H410 | ||
Precautionary statements |
P201, P202, P210, P233, P240, P241, P242, P243, P260, P271, P273, P280, P281, P284, P303+P361+P353, P304+P340, P308+P313, P310, P320, P370+P378, P391, P403+P233, P403+P235, P405, P501 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 4 °C (39 °F; 277 K) | ||
Autoignition temperature |
60 °C (140 °F; 333 K) | ||
| Explosive limits | 2–34% | ||
| Lethal dose or concentration (LD, LC): | |||
LC50 (median concentration) |
266 ppm (cat, 30 min) 35 ppm (rabbit, 30 min) 94 ppm (mouse, 30 min) 10 ppm (mouse, 10 min)[3] | ||
LCLo (lowest published) |
360 ppm (dog, 90 min) 30 ppm (human, 30 min) 42 ppm (rabbit, 30 min) 7 ppm (mouse, 30 min)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 0.001 ppm (0.007 mg/m3)[1] | ||
REL (Recommended) |
TWA 0.001 ppm (0.007 mg/m3)[1] | ||
IDLH (Immediate danger) |
Ca [2 ppm][1] | ||
| Safety data sheet (SDS) | ICSC 0064 | ||
| Related compounds | |||
Related metal carbonyls |
Iron pentacarbonyl Dicobalt octacarbonyl | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Structure and bonding
In nickel tetracarbonyl, the oxidation state for nickel is assigned as zero. The formula conforms to 18-electron rule. The molecule is tetrahedral, with four carbonyl (carbon monoxide) ligands. Electron diffraction studies have been performed on this molecule, and the Ni–C and C–O distances have been calculated to be 1.838(2) and 1.141(2) angstroms respectively.[5]
Preparation
Ni(CO)4 was first synthesised in 1890 by Ludwig Mond by the direct reaction of nickel metal with carbon monoxide.[6] This pioneering work foreshadowed the existence of many other metal carbonyl compounds, including those of vanadium, chromium, manganese, iron, and cobalt. It was also applied industrially to the purification of nickel by the end of the 19th century.[7]
At 323 K (50 °C; 122 °F), carbon monoxide is passed over impure nickel. The optimal rate occurs at 130 °C.[8]
Laboratory routes
Ni(CO)4 is not readily available commercially. It is conveniently generated in the laboratory by carbonylation of commercially available bis(cyclooctadiene)nickel(0).[9] It can also be prepared by reduction of ammoniacal solutions of nickel sulfate with sodium dithionite under an atmosphere of CO.[10]
Reactions

Thermal decarbonylation
On moderate heating, Ni(CO)4 decomposes to carbon monoxide and nickel metal. Combined with the easy formation from CO and even very impure nickel, this decomposition is the basis for the Mond process for the purification of nickel or plating onto surfaces. Thermal decomposition commences near 180 °C and increases at higher temperature.[8]
Reactions with nucleophiles and reducing agents
Like other low-valent metal carbonyls, Ni(CO)4 is susceptible to attack by nucleophiles. Attack can occur at nickel center, resulting in displacement of CO ligands, or at CO. Thus, donor ligands such as triphenylphosphine react to give Ni(CO)3(PPh3) and Ni(CO)2(PPh3)2. Bipyridine and related ligands behave similarly.[11] The monosubstitution of nickel tetracarbonyl with other ligands can be used to determine the Tolman electronic parameter, a measure of the electron donating or withdrawing ability of a given ligand.
2.svg.png.webp) Structure of Ni(PPh3)2(CO)2.
Structure of Ni(PPh3)2(CO)2.
Treatment with hydroxides gives clusters such as [Ni5(CO)12]2− and [Ni6(CO)12]2−. These compounds can also be obtained by reduction of nickel carbonyl.
Thus, treatment of Ni(CO)4 with carbon nucleophiles (Nu−) results in acyl derivatives such as [Ni(CO)3C(O)Nu)]−.[12]
Reactions with electrophiles and oxidizing agents
Nickel carbonyl can be oxidized. Chlorine oxidizes nickel carbonyl into NiCl2, releasing CO gas. Other halogens behave analogously. This reaction provides a convenient method for precipitating the nickel portion of the toxic compound.
Reactions of Ni(CO)4 with alkyl and aryl halides often result in carbonylated organic products. Vinylic halides, such as PhCH=CHBr, are converted to the unsaturated esters upon treatment with Ni(CO)4 followed by sodium methoxide. Such reactions also probably proceed via oxidative addition. Allylic halides give the π-allylnickel compounds, such as (allyl)2Ni2Cl2:[13]
- 2 Ni(CO)4 + 2 ClCH2CH=CH2 → Ni2(μ-Cl)2(η3-C3H5)2 + 8 CO
Toxicology and safety considerations
The hazards of Ni(CO)4 are far greater than that implied by its CO content, reflecting the effects of the nickel if released in the body. Nickel carbonyl may be fatal if absorbed through the skin or more likely, inhaled due to its high volatility. Its LC50 for a 30-minute exposure has been estimated at 3 ppm, and the concentration that is immediately fatal to humans would be 30 ppm. Some subjects exposed to puffs up to 5 ppm described the odour as musty or sooty, but because the compound is so exceedingly toxic, its smell provides no reliable warning against a potentially fatal exposure.[14]
The vapours of Ni(CO)4 can autoignite. The vapor decomposes quickly in air, with a half-life of about 40 seconds.[15]
Nickel carbonyl poisoning is characterized by a two-stage illness. The first consists of headaches and chest pain lasting a few hours, usually followed by a short remission. The second phase is a chemical pneumonitis which starts after typically 16 hours with symptoms of cough, breathlessness and extreme fatigue. These reach greatest severity after four days, possibly resulting in death from cardiorespiratory or acute kidney injury. Convalescence is often extremely protracted, often complicated by exhaustion, depression and dyspnea on exertion. Permanent respiratory damage is unusual. The carcinogenicity of Ni(CO)4 is a matter of debate, but is presumed to be significant.
It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.[16]
In popular culture
"Requiem for the Living" (1978), an episode of Quincy, M.E., features a poisoned, dying crime lord who asks Dr. Quincy to autopsy his still-living body. Quincy identifies the poison—nickel carbonyl.
In the novella Amanda Morgan by Gordon R. Dickson, the remaining inhabitants of a mostly evacuated village resist an occupying military force by directing the exhaust from a poorly-tuned internal combustion engine onto a continually renewed "waste heap" of powdered nickel outside a machine shop (under the guise of civilian business) in order to eliminate the occupiers, at the cost of their own lives.
In chapter 199 of the manga Dr. Stone, a machine is made that purifies nickel via the Mond Process. It is mentioned that the process creates a "fatal toxin" (nickel carbonyl).
References
- NIOSH Pocket Guide to Chemical Hazards. "#0444". National Institute for Occupational Safety and Health (NIOSH).
- Nickel tetracarbonyl, carcinogenicity
- "Nickel carbonyl". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- The Merck Index (7th ed.). Merck.
- Hedberg, L.; Iijima, T.; Hedberg, K. (1979). "Nickel tetracarbonyl, Ni(CO)4. I. Molecular Structure by Gaseous Electron Diffraction. II. Refinement of Quadratic Force Field". The Journal of Chemical Physics. 70 (7): 3224–3229. Bibcode:1979JChPh..70.3224H. doi:10.1063/1.437911.
- Mond, L.; Langer, C.; Quincke, F. (1890). "Action of Carbon Monoxide on Nickel". J. Chem. Soc. Trans. 57: 749–753. doi:10.1039/CT8905700749.
- "The Extraction of Nickel from its Ores by the Mond Process". Nature. 59 (1516): 63–64. 1898. Bibcode:1898Natur..59...63.. doi:10.1038/059063a0.
- Lascelles, K.; Morgan, L. G.; Nicholls, D.; Beyersmann, D. "Nickel Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_235.pub2.
- Jolly, P. W. (1982). "Nickel Tetracarbonyl". In Abel, Edward W.; Stone, F. Gordon A.; Wilkinson, Geoffrey (eds.). Comprehensive Organometallic Chemistry. Vol. I. Oxford: Pergamon Press. ISBN 0-08-025269-9.
- F. Seel (1963). "Nickel Carbonyl". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry. Vol. 2 (2nd ed.). NY: Academic Press. pp. 1747–1748.
- Elschenbroich, C.; Salzer, A. (1992). Organometallics: A Concise Introduction (2nd ed.). Weinheim: Wiley-VCH. ISBN 3-527-28165-7.
- Pinhas, A. R. (2003). "Tetracarbonylnickel". Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rt025m. ISBN 0471936235.
- Semmelhack, M. F.; Helquist, P. M. (1972). "Reaction of Aryl Halides with π-Allylnickel Halides: Methallylbenzene". Organic Syntheses. 52: 115.; Collective Volume, vol. 6, p. 722
- Board on Environmental Studies and Toxicology (2008). "Nickel Carbonyl: Acute Exposure Guideline Levels". Acute Exposure Guideline Levels for Selected Airborne Chemicals. Vol. 6. National Academies Press. pp. 213–259. doi:10.17226/12018. ISBN 978-0-309-11213-0. PMID 25032325.
- Stedman, D. H.; Hikade, D. A.; Pearson, R. Jr.; Yalvac, E. D. (1980). "Nickel Carbonyl: Decomposition in Air and Related Kinetic Studies". Science. 208 (4447): 1029–1031. Bibcode:1980Sci...208.1029S. doi:10.1126/science.208.4447.1029. PMID 17779026. S2CID 31344783.
- "40 C.F.R.: Appendix A to Part 355—The List of Extremely Hazardous Substances and Their Threshold Planning Quantities" (PDF) (July 1, 2008 ed.). Government Printing Office. Archived from the original (PDF) on February 25, 2012. Retrieved October 29, 2011.
{{cite journal}}: Cite journal requires|journal=(help)
Further reading
- Shi, Z. (1991). "Nickel Carbonyl: Toxicity and Human Health". Science of the Total Environment. 148 (2–3): 293–298. doi:10.1016/0048-9697(94)90406-5. PMID 8029705.
- Sunderman, F. W. (1989). "A Pilgrimage into the Archives of Nickel Toxicology". Annals of Clinical and Laboratory Science. 19 (1): 1–16. PMID 2644888.
- Armit, H. W. (1907). "The Toxicology of Nickel Carbonyl. Part I". Journal of Hygiene. 7 (4): 525–551. doi:10.1017/S0022172400033507. PMC 2236193. PMID 20474327.
- Armit, H. W. (1908). "The Toxicology of Nickel Carbonyl. Part II". Journal of Hygiene. 8 (5): 565–610. doi:10.1017/S0022172400015989. PMC 2167169. PMID 20474374.
- Barceloux, D. G.; Barceloux, Donald (1999). "Nickel". Clinical Toxicology. 37 (2): 239–258. doi:10.1081/CLT-100102423. PMID 10382559.