Tetrahydrocannabinol
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers,[9] the term THC usually refers to the Delta-9-THC isomer with chemical name (−)-trans-Δ9-tetrahydrocannabinol. THC is a lipid found in cannabis[10] and, like most pharmacologically active secondary metabolites of plants, it is assumed to be involved in the plant's evolutionary adaptation, putatively against insect predation, ultraviolet light, and environmental stress.[11][12][13]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Marinol, Syndros |
| Other names | (6aR,10aR)-delta-9-Tetrahydrocannabinol; (−)-trans-Δ9-Tetrahydrocannabinol; THC |
| License data |
|
| Dependence liability | 8–10% (Relatively low risk of tolerance)[1] |
| Routes of administration | Oral, local/topical, transdermal, sublingual, inhaled |
| Drug class | cannabinoid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 10–35% (inhalation), 6–20% (oral)[3] |
| Protein binding | 97–99%[3][4][5] |
| Metabolism | Mostly hepatic by CYP2C[3] |
| Elimination half-life | 1.6–59 h,[3] 25–36 h (orally administered dronabinol) |
| Excretion | 65–80% (feces), 20–35% (urine) as acid metabolites[3] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.153.676 |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.469 g·mol−1 |
| 3D model (JSmol) | |
| Specific rotation | −152° (ethanol) |
| Boiling point | 155–157°C @ 0.05mmHg,[6] 157–160°C @ 0.05mmHg[7] |
| Solubility in water | 0.0028 mg/mL (23 °C)[8] |
SMILES
| |
InChI
| |
| | |
THC, along with its double bond isomers and their stereoisomers,[14] is one of only three cannabinoids scheduled by the UN Convention on Psychotropic Substances (the other two are dimethylheptylpyran and parahexyl). It was listed under Schedule I in 1971, but reclassified to Schedule II in 1991 following a recommendation from the WHO. Based on subsequent studies, the WHO has recommended the reclassification to the less-stringent Schedule III.[15] Cannabis as a plant is scheduled by the Single Convention on Narcotic Drugs (Schedule I and IV). It is specifically still listed under Schedule I by US federal law[16] under the Controlled Substances Act for having "no accepted medical use" and "lack of accepted safety". However, dronabinol, a pharmaceutical form of THC, has been approved by the FDA as an appetite stimulant for people with AIDS and an antiemetic for people receiving chemotherapy under the trade names Marinol and Syndros.[17] The pharmaceutical formulation dronabinol is an oily and viscous resin provided in capsules available by prescription in the United States, Canada, Germany, and New Zealand.[18]
Delta-9-tetrahydrocannabinol (Δ9-THC), better known as THC, is the marijuana plant's primary component for causing psychoactive effects. THC was first discovered and isolated by Israeli chemist Raphael Mechoulam in Israel in 1964. It was found that, when smoked, tetrahydrocannabinol is absorbed into the bloodstream and travels to the brain, attaching itself to the naturally occurring endocannabinoid receptors located in the cerebral cortex, cerebellum, and basal ganglia. These are the parts of the brain responsible for thinking, memory, pleasure, coordination and movement.[19]
Medical uses
THC is an active ingredient in Nabiximols, a specific extract of Cannabis that was approved as a botanical drug in the United Kingdom in 2010 as a mouth spray for people with multiple sclerosis to alleviate neuropathic pain, spasticity, overactive bladder, and other symptoms.[20][21] Nabiximols (as Sativex) is available as a prescription drug in Canada.[22] In 2021, Nabiximols was approved for medical use in Ukraine.[23]
Pharmacology
Mechanism of action
The actions of Delta-9-THC result from its partial agonist activity at the cannabinoid receptor CB1 (Ki = 40.7 nM[24]), located mainly in the central nervous system, and the CB2 receptor (Ki = 36 nM[24]), mainly expressed in cells of the immune system.[25] The psychoactive effects of THC are primarily mediated by the activation of cannabinoid receptors, which result in a decrease in the concentration of the second messenger molecule cAMP through inhibition of adenylate cyclase.[26] The presence of these specialized cannabinoid receptors in the brain led researchers to the discovery of endocannabinoids, such as anandamide and 2-arachidonoyl glyceride (2-AG).
THC is a lipophilic molecule[27] and may bind non-specifically to a variety of entities in the brain and body, such as adipose tissue (fat).[28][29] THC, as well as other cannabinoids that contain a phenol group, possess mild antioxidant activity sufficient to protect neurons against oxidative stress, such as that produced by glutamate-induced excitotoxicity.[25]
THC targets receptors in a manner far less selective than endocannabinoid molecules released during retrograde signaling, as the drug has a relatively low cannabinoid receptor affinity. THC is also limited in its efficacy compared to other cannabinoids due to its partial agonistic activity, as THC appears to result in greater downregulation of cannabinoid receptors than endocannabinoids. Furthermore, in populations of low cannabinoid receptor density, THC may even act to antagonize endogenous agonists that possess greater receptor efficacy. However while THC's pharmacodynamic tolerance may limit the maximal effects of certain drugs, evidence suggests that this tolerance mitigates undesirable effects, thus enhancing the drug's therapeutic window.[30]
Pharmacokinetics
THC is metabolized mainly to 11-OH-THC by the body. This metabolite is still psychoactive and is further oxidized to 11-nor-9-carboxy-THC (THC-COOH). In animals, more than 100 metabolites could be identified, but 11-OH-THC and THC-COOH are the dominating metabolites.[31] Metabolism occurs mainly in the liver by cytochrome P450 enzymes CYP2C9, CYP2C19, CYP2D6, and CYP3A4.[32][33] More than 55% of THC is excreted in the feces and ≈20% in the urine. The main metabolite in urine is the ester of glucuronic acid and 11-OH-THC and free THC-COOH. In the feces, mainly 11-OH-THC was detected.[34]
Physical and chemical properties
Discovery and structure identification
Cannabidiol was isolated and identified from Cannabis sativa in 1940,[35] and THC was isolated and its structure elucidated by synthesis in 1964.[36][37]
Solubility
As with many aromatic terpenoids, THC has a very low solubility in water, but good solubility in lipids and most organic solvents, specifically hydrocarbons and alcohols.[8]
Unknown lethal dose
The median lethal dose of THC in humans is not known. A 1972 study gave up to 9000 mg/kg of THC to dogs and monkeys without any lethal effects. Some rats died within 72 hours after a dose of up to 3600 mg/kg.[38]
Total synthesis
A total synthesis of the compound was reported in 1965; that procedure called for the intramolecular alkyl lithium attack on a starting carbonyl to form the fused rings, and a tosyl chloride mediated formation of the ether.[39]
Biosynthesis
In the Cannabis plant, THC occurs mainly as tetrahydrocannabinolic acid (THCA, 2-COOH-THC). Geranyl pyrophosphate and olivetolic acid react, catalysed by an enzyme to produce cannabigerolic acid,[40] which is cyclized by the enzyme THC acid synthase to give THCA. Over time, or when heated, THCA is decarboxylated, producing THC. The pathway for THCA biosynthesis is similar to that which produces the bitter acid humulone in hops.[41][42] It can also be produced in genetically modified yeast.[43]
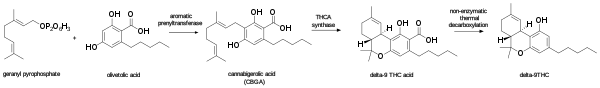 Biosynthesis of THC
Biosynthesis of THC
Detection in body fluids
THC and its 11-OH-THC and THC-COOH metabolites can be detected and quantified in blood, urine, hair, oral fluid or sweat using a combination of immunoassay and chromatographic techniques as part of a drug use testing program or in a forensic investigation.[44][45][46]
Detection in breath
Recreational use of cannabis is legal in many parts of North America, increasing the demand for THC monitoring methods in both personal and law enforcement uses.[47] Breath sampling as a noninvasive method is in development to detect THC, which is difficult to quantify in breath samples.[47] Scientists and industry are commercializing various types of breath analyzers to monitor THC in breath.[48]
History
THC was first isolated and elucidated in 1969 by Raphael Mechoulam and Yechiel Gaoni at the Weizmann Institute of Science in Israel.[36][49][50]
In 2003, the World Health Organization Expert Committee on Drug Dependence recommended transferring THC to Schedule IV of the Convention, citing its medical uses and low abuse and addiction potential.[51] The Agriculture Improvement Act of 2018 allows any hemp-derived product not exceeding 0.3% Δ-9 THC to be sold legally in the US. Since the law counted only Δ-9 THC, Δ-8 THC was considered legal to sell under the farm bill and is widely available online.[52]
Society and culture
Comparisons with medical cannabis
| Part of a series on |
| Cannabis |
|---|
 |
|
Female cannabis plants contain at least 113 cannabinoids,[53] including cannabidiol (CBD), thought to be the major anticonvulsant that helps people with multiple sclerosis,[54] and cannabichromene (CBC), an anti-inflammatory which may contribute to the pain-killing effect of cannabis.[55]
Regulation in Canada
As of October 2018 when recreational use of cannabis was legalized in Canada, some 220 dietary supplements and 19 veterinary health products containing not more than 10 parts per million of THC extract were approved with general health claims for treating minor conditions.[22]
Research
The status of THC as an illegal drug in most countries imposes restrictions on research material supply and funding, such as in the United States where the National Institute on Drug Abuse and Drug Enforcement Administration continue to control the sole federally-legal source of cannabis for researchers. Despite an August 2016 announcement that licenses would be provided to growers for supplies of medical marijuana, no such licenses were ever issued, despite dozens of applications.[56] Although cannabis is legalized for medical uses in more than half of the states of the United States, no products have been approved for federal commerce by the Food and Drug Administration, a status that limits cultivation, manufacture, distribution, clinical research, and therapeutic applications.[57]
In April 2014, the American Academy of Neurology found evidence supporting the effectiveness of the cannabis extracts in treating certain symptoms of multiple sclerosis and pain, but there was insufficient evidence to determine effectiveness for treating several other neurological diseases.[58] A 2015 review confirmed that medical marijuana was effective for treating spasticity and chronic pain, but caused numerous short-lasting adverse events, such as dizziness.[59]
Multiple sclerosis symptoms
- Spasticity. Based on the results of 3 high quality trials and 5 of lower quality, oral cannabis extract was rated as effective, and THC as probably effective, for improving people's subjective experience of spasticity. Oral cannabis extract and THC both were rated as possibly effective for improving objective measures of spasticity.[58][59]
- Centrally mediated pain and painful spasms. Based on the results of 4 high quality trials and 4 low quality trials, oral cannabis extract was rated as effective, and THC as probably effective in treating central pain and painful spasms.[58]
- Bladder dysfunction. Based on a single high quality study, oral cannabis extract and THC were rated as probably ineffective for controlling bladder complaints in multiple sclerosis[58]
Neurodegenerative disorders
- Huntington disease. No reliable conclusion could be drawn regarding the effectiveness of THC or oral cannabis extract in treating the symptoms of Huntington disease as the available trials were too small to reliably detect any difference[58]
- Parkinson's disease. Based on a single study, oral CBD extract was rated probably ineffective in treating levodopa-induced dyskinesia in Parkinson's disease.[58]
- Alzheimer's disease. A 2009 Cochrane Review found insufficient evidence to conclude whether cannabis products have any utility in the treatment of Alzheimer's disease.[60]
Other neurological disorders
- Tourette syndrome. The available data was determined to be insufficient to allow reliable conclusions to be drawn regarding the effectiveness of oral cannabis extract or THC in controlling tics.[58]
- Cervical dystonia. Insufficient data was available to assess the effectiveness of oral cannabis extract of THC in treating cervical dystonia.[58]
Potential for toxicity
Preliminary research indicates that prolonged exposure to high doses of THC may interfere with chromosomal stability, which may be hereditary as a factor affecting cell instability and cancer risk. The carcinogenicity of THC in the studied populations of so-called "heavy users" remains dubious due to various confounding variables, most significantly concurrent tobacco use.[61]
See also
- Cannabinoid hyperemesis syndrome (CHS)
- Cannabinoids
- 11-Hydroxy-THC, metabolite of THC
- Anandamide, 2-Arachidonoylglycerol, endogenous cannabinoid agonists
- Cannabidiol (CBD)
- Cannabinol (CBN), a metabolite of THC
- Delta-8-Tetrahydrocannabinol, a double bond isomer of THC
- Dimethylheptylpyran
- Dronabinol, the name of THC-based pharmaceutical (INN)
- HU-210, WIN 55,212-2, JWH-133, synthetic cannabinoid agonists (neocannabinoids)
- Nabilone, a novel synthetic cannabinoid analog (neocannabinoid)
- Parahexyl
- Tetrahydrocannabinolic acid (THCA), the biosynthetic precursor for THC
- Tetrahydrocannabiphorol, the heptyl homologue
- List of investigational analgesics
- Medical cannabis
- Dronabinol
- Epidiolex (prescription form of purified cannabidiol derived from hemp used for treating some rare neurological diseases)
- Sativex
- Effects of cannabis
- Vaping-associated pulmonary injury
- War on Drugs
References
- Marlowe DB (December 2010). "The Facts On Marijuana" (PDF). NADCP.
Based upon several nationwide epidemiological studies, marijuana's dependence liability has been reliably determined to be 8 to 10 percent.
- "Marinol" (PDF). Food and Drug Administration. Archived from the original (PDF) on 2014-05-13. Retrieved 2014-03-14.
- Grotenhermen F (2003). "Pharmacokinetics and pharmacodynamics of cannabinoids". Clinical Pharmacokinetics. 42 (4): 327–60. doi:10.2165/00003088-200342040-00003. PMID 12648025. S2CID 25623600.
- The Royal Pharmaceutical Society of Great Britain (2006). "Cannabis". In Sweetman SC (ed.). Martindale: The Complete Drug Reference: Single User (35th ed.). Pharmaceutical Press. ISBN 978-0-85369-703-9.
- "Tetrahydrocannabinol – Compound Summary". National Center for Biotechnology Information. PubChem. Retrieved 12 January 2014.
Dronabinol has a large apparent volume of distribution, approximately 10 L/kg, because of its lipid solubility. The plasma protein binding of dronabinol and its metabolites is approximately 97%.
- Gaoni Y, Mechoulam R (April 1964). "Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish". Journal of the American Chemical Society. 86 (8): 1646–47. doi:10.1021/ja01062a046.
- Adams R, Cain CK, McPhee WD, Wearn RB (August 1941). "Structure of Cannabidiol. XII. Isomerization to Tetrahydrocannabinols". Journal of the American Chemical Society. 63 (8): 2209–13. doi:10.1021/ja01853a052.
- Garrett ER, Hunt CA (July 1974). "Physiochemical properties, solubility, and protein binding of delta9-tetrahydrocannabinol". Journal of Pharmaceutical Sciences. 63 (7): 1056–64. doi:10.1002/jps.2600630705. PMID 4853640.
- "THC Chemistry by Alexander Shulgin - January 21, 1995". www.druglibrary.org. Retrieved 2020-11-12.
- Firn, Richard (2010). Nature's Chemicals. Oxford: Biology.
- Pate DW (1994). "Chemical ecology of Cannabis". Journal of the International Hemp Association. 2 (29): 32–37.
- Pate DW (1983). "Possible role of ultraviolet radiation in evolution of Cannabis chemotypes". Economic Botany. 37 (4): 396–405. doi:10.1007/BF02904200. S2CID 35727682.
- Lydon J, Teramura AH, Coffman CB (August 1987). "UV-B radiation effects on photosynthesis, growth and cannabinoid production of two Cannabis sativa chemotypes". Photochemistry and Photobiology. 46 (2): 201–06. doi:10.1111/j.1751-1097.1987.tb04757.x. PMID 3628508. S2CID 7938905.
- Mazzoccanti G, Ismail OH, D'Acquarica I, Villani C, Manzo C, Wilcox M, et al. (November 2017). "Cannabis through the looking glass: chemo- and enantio-selective separation of phytocannabinoids by enantioselective ultra high performance supercritical fluid chromatography". Chemical Communications. 53 (91): 12262–65. doi:10.1039/C7CC06999E. hdl:11573/1016698. PMID 29072720.
- "The UN Drug Control Conventions". 8 October 2015.
- "Drug Schedules; Schedule 1". United States Drug Enforcement Administration. US Drug Enforcement Administration, Department of Justice. 1 December 2017. Retrieved 14 January 2018.
{{cite web}}: CS1 maint: url-status (link) - "Marinol (Dronabinol)" (PDF). US Food and Drug Administration. September 2004. Retrieved 14 January 2018.
- "Can Dronabinol Help Treat Sleep Apnea?". www.healthcentral.com. Retrieved 2021-03-28.
- "What Is THC? – Herbies". herbiesheadshop.com. Retrieved 2020-10-28.
- "Sativex Oromucosal Spray – Summary of Product Characteristics". UK Electronic Medicines Compendium. March 2015.
- Multiple Sclerosis Trust. October 2014 Sativex (nabiximols) – factsheet
- "Health products containing cannabis or for use with cannabis: Guidance for the Cannabis Act, the Food and Drugs Act, and related regulations". Government of Canada. 11 July 2018. Retrieved 19 October 2018.
- "В Україні легалізували використання медичного канабісу, але не всього" [In Ukraine, some medical cannabis has been legalized, but not all]. УП.Життя (UP.Life) (in Ukrainian). 9 April 2021.
- Bow EW, Rimoldi JM (28 June 2016). "The Structure–Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation". Perspectives in Medicinal Chemistry. 8: 17–39. doi:10.4137/PMC.S32171. PMC 4927043. PMID 27398024.
- Pertwee RG (April 2006). "The pharmacology of cannabinoid receptors and their ligands: an overview". International Journal of Obesity. 30 (Suppl 1): S13–S18. doi:10.1038/sj.ijo.0803272. PMID 16570099.
- Elphick MR, Egertová M (March 2001). "The neurobiology and evolution of cannabinoid signalling". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 356 (1407): 381–408. doi:10.1098/rstb.2000.0787. PMC 1088434. PMID 11316486.
- Rashidi H, Akhtar MT, van der Kooy F, Verpoorte R, Duetz WA (November 2009). "Hydroxylation and further oxidation of delta9-tetrahydrocannabinol by alkane-degrading bacteria". Applied and Environmental Microbiology. 75 (22): 7135–41. Bibcode:2009ApEnM..75.7135R. doi:10.1128/AEM.01277-09. PMC 2786519. PMID 19767471.
Δ9-THC and many of its derivatives are highly lipophilic and poorly water soluble. Calculations of the n-octanol-water partition coefficient (Ko/w) of Δ9-THC at neutral pH vary between 6,000, using the shake flask method, and 9.44 × 106, by reverse-phase high-performance liquid chromatography estimation.
- Ashton CH (February 2001). "Pharmacology and effects of cannabis: a brief review". The British Journal of Psychiatry. 178 (2): 101–06. doi:10.1192/bjp.178.2.101. PMID 11157422.
Because they are extremely lipid soluble, cannabinoids accumulate in fatty tissues, reaching peak concentrations in 4–5 days. They are then slowly released back into other body compartments, including the brain. ... Within the brain, THC and other cannabinoids are differentially distributed. High concentrations are reached in neocortical, limbic, sensory and motor areas.
- Huestis MA (August 2007). "Human cannabinoid pharmacokinetics". Chemistry & Biodiversity. 4 (8): 1770–804. doi:10.1002/cbdv.200790152. PMC 2689518. PMID 17712819.
THC is highly lipophilic and initially taken up by tissues that are highly perfused, such as the lung, heart, brain, and liver.
- Pertwee RG (January 2008). "The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin". British Journal of Pharmacology. 153 (2): 199–215. doi:10.1038/sj.bjp.0707442. PMC 2219532. PMID 17828291.
- Aizpurua-Olaizola O, Zarandona I, Ortiz L, Navarro P, Etxebarria N, Usobiaga A (April 2017). "Simultaneous quantification of major cannabinoids and metabolites in human urine and plasma by HPLC-MS/MS and enzyme-alkaline hydrolysis". Drug Testing and Analysis. 9 (4): 626–33. doi:10.1002/dta.1998. PMID 27341312.
- Qian Y, Gurley BJ, Markowitz JS (2019). "The Potential for Pharmacokinetic Interactions Between Cannabis Products and Conventional Medications". Journal of Clinical Psychopharmacology. 39 (5): 462–71. doi:10.1097/JCP.0000000000001089. PMID 31433338. S2CID 201118659.
- Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I (March 2007). "Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes". Life Sciences. 80 (15): 1415–19. doi:10.1016/j.lfs.2006.12.032. PMID 17303175.
- Huestis MA (2005). "Pharmacokinetics and Metabolism of the Plant Cannabinoids, Δ9-Tetrahydrocannibinol, Cannabidiol and Cannabinol". Pharmacokinetics and Metabolism of the Plant Cannabinoids, Δ9-Tetrahydrocannabinol, Cannabidiol and Cannabinol. Cannabinoids. Handbook of Experimental Pharmacology. Vol. 168. pp. 657–90. doi:10.1007/3-540-26573-2_23. ISBN 978-3-540-22565-2. PMID 16596792.
- Adams R, Hunt M, Clark JH (1940). "Structure of Cannabidiol, a Product Isolated from the Marihuana Extract of Minnesota Wild Hemp". Journal of the American Chemical Society. 62: 196–200. doi:10.1021/ja01858a058.
- Gaoni Y, Mechoulam R (1964). "Isolation, structure and partial synthesis of an active constituent of hashish". Journal of the American Chemical Society. 86 (8): 1646–47. doi:10.1021/ja01062a046.
- Mechoulam R (June 1970). "Marihuana chemistry". Science. 168 (3936): 1159–66. Bibcode:1970Sci...168.1159M. doi:10.1126/science.168.3936.1159. PMID 4910003.
- Thompson GR, Rosenkrantz H, Schaeppi UH, Braude MC (July 1973). "Comparison of acute oral toxicity of cannabinoids in rats, dogs and monkeys". Toxicology and Applied Pharmacology. 25 (3): 363–72. doi:10.1016/0041-008X(73)90310-4. PMID 4199474.
In dogs and monkeys, single oral doses of Δ9-THC and Δ8-THC between 3000 and 9000/mg/kg were nonlethal.
- Mechoulam R, Gaoni Y (July 1965). "A Total Synthesis of Dl-Delta-1-Tetrahydrocannabinol, the Active Constituent of Hashish". Journal of the American Chemical Society. 87 (14): 3273–75. doi:10.1021/ja01092a065. PMID 14324315.
- Fellermeier M, Zenk MH (May 1998). "Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol". FEBS Letters. 427 (2): 283–85. doi:10.1016/S0014-5793(98)00450-5. PMID 9607329.
- Marks MD, Tian L, Wenger JP, Omburo SN, Soto-Fuentes W, He J, et al. (2009). "Identification of candidate genes affecting Delta9-tetrahydrocannabinol biosynthesis in Cannabis sativa". Journal of Experimental Botany. 60 (13): 3715–26. doi:10.1093/jxb/erp210. PMC 2736886. PMID 19581347.
- Baker PB, Taylor BJ, Gough TA (June 1981). "The tetrahydrocannabinol and tetrahydrocannabinolic acid content of cannabis products". The Journal of Pharmacy and Pharmacology. 33 (6): 369–72. doi:10.1111/j.2042-7158.1981.tb13806.x. PMID 6115009. S2CID 30412893.
- Luo X, Reiter MA, d'Espaux L, Wong J, Denby CM, Lechner A, et al. (March 2019). "Complete biosynthesis of cannabinoids and their unnatural analogues in yeast" (PDF). Nature. 567 (7746): 123–26. Bibcode:2019Natur.567..123L. doi:10.1038/s41586-019-0978-9. PMID 30814733. S2CID 71147445.
- Schwilke EW, Schwope DM, Karschner EL, Lowe RH, Darwin WD, Kelly DL, et al. (December 2009). "Delta9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC". Clinical Chemistry. 55 (12): 2180–89. doi:10.1373/clinchem.2008.122119. PMC 3196989. PMID 19833841.
- Röhrich J, Schimmel I, Zörntlein S, Becker J, Drobnik S, Kaufmann T, et al. (May 2010). "Concentrations of delta9-tetrahydrocannabinol and 11-nor-9-carboxytetrahydrocannabinol in blood and urine after passive exposure to Cannabis smoke in a coffee shop". Journal of Analytical Toxicology. 34 (4): 196–203. doi:10.1093/jat/34.4.196. PMID 20465865.
- Baselt R (2011). Disposition of Toxic Drugs and Chemicals in Man (9th ed.). Seal Beach, CA: Biomedical Publications. pp. 1644–48.
- Alicia Wallace (January 2, 2020). "Testing drivers for cannabis is hard. Here's why". CNN Business. Retrieved 26 February 2020.
- Mirzaei H, O'Brien A, Tasnim N, Ravishankara A, Tahmooressi H, Hoorfar M (May 2020). "Topical review on monitoring tetrahydrocannabinol in breath". Journal of Breath Research. 14 (3): 034002. Bibcode:2020JBR....14c4002M. doi:10.1088/1752-7163/ab6229. PMID 31842004. S2CID 209388839.
- "Interview with the winner of the first ECNP Lifetime Achievement Award: Raphael Mechoulam, Israel". February 2007. Archived from the original on 2011-04-30.
- Geller T (2007). "Cannabinoids: A Secret History". Chemical Heritage Newsmagazine. 25 (2). Archived from the original on 19 June 2008.
- "WHO Expert Committee on Drug Dependence". World Health Organization. Archived from the original on January 7, 2005. Retrieved 12 January 2014.
- "Delta 8 THC: Everything You Need To Know". LA Weekly. 2020-07-09. Retrieved 2020-07-14.
- Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, et al. (February 2016). "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products. 79 (2): 324–31. doi:10.1021/acs.jnatprod.5b00949. PMID 26836472.
- Pickens JT (April 1981). "Sedative activity of cannabis in relation to its delta'-trans-tetrahydrocannabinol and cannabidiol content". British Journal of Pharmacology. 72 (4): 649–56. doi:10.1111/j.1476-5381.1981.tb09145.x. PMC 2071638. PMID 6269680.
- Morales P, Hurst DP, Reggio PH (2017). "Molecular Targets of the Phytocannabinoids: A Complex Picture". Progress in the Chemistry of Organic Natural Products. 103: 103–31. doi:10.1007/978-3-319-45541-9_4. ISBN 978-3-319-45539-6. PMC 5345356. PMID 28120232.
- "Medical Marijuana". Multidisciplinary Association for Psychedelic Studies. Retrieved 12 January 2014.
- Mead A (May 2017). "The legal status of cannabis (marijuana) and cannabidiol (CBD) under U.S. law". Epilepsy & Behavior. 70 (Pt B): 288–91. doi:10.1016/j.yebeh.2016.11.021. PMID 28169144.
- Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, Gloss D (April 2014). "Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology". Neurology. 82 (17): 1556–63. doi:10.1212/WNL.0000000000000363. PMC 4011465. PMID 24778283.
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, et al. (2015). "Cannabinoids for Medical Use: A Systematic Review and Meta-analysis". JAMA. 313 (24): 2456–73. doi:10.1001/jama.2015.6358. PMID 26103030.
- Krishnan S, Cairns R, Howard R (April 2009). Krishnan S (ed.). "Cannabinoids for the treatment of dementia". The Cochrane Database of Systematic Reviews (2): CD007204. doi:10.1002/14651858.CD007204.pub2. PMC 7197039. PMID 19370677.
- Reece AS, Hulse GK (July 2016). "Chromothripsis and epigenomics complete causality criteria for cannabis- and addiction-connected carcinogenicity, congenital toxicity and heritable genotoxicity". Mutation Research. 789: 15–25. doi:10.1016/j.mrfmmm.2016.05.002. PMID 27208973.