Phenserine
Phenserine (also known as (-)-phenserine or (-)-eseroline phenylcarbamate) is a synthetic drug which has been investigated as a medication to treat Alzheimer's disease (AD), as the drug exhibits neuroprotective and neurotrophic effects.
 | |
| Clinical data | |
|---|---|
| Other names | (-)-Phenserine, (-)-Eseroline phenylcarbamate, N-phenylcarbamoyleseroline, N-phenylcarbamoyl eseroline |
| Routes of administration | By mouth |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~100% [1] |
| Metabolism | liver |
| Metabolites | (−)-N1-norphenserine, (−)-N8-norphenserine, (−)-N1,N8-bisnorphenserine |
| Elimination half-life | 12.6 minutes |
| Duration of action | 8.25 hours [2] |
| Excretion | renal or hepatic clearance [2] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.162.417 |
| Chemical and physical data | |
| Formula | C20H23N3O2 |
| Molar mass | 337.4155 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 150 °C (302 °F) |
SMILES
| |
InChI
| |
The research of phenserine, initially patented by the National Institute on Aging (NIA),[3] has been suspended since phase III of clinical trials in 2006, conducted right after the drug licenses were issued.[4] The abandonment of the clinical trials led to disapproval by FDA.[5] The retrospective meta-analysis of the phenserine research proposed that its clinical invalidation was arisen from methodological issues that were not impeccably settled before proceeding to the subsequent clinical phases.[5][6]
Phenserine was introduced as an inhibitor of acetylcholinesterase (AChE) and demonstrated significant alleviation in numerous neuropathological manifestations, improving cognitive functions of the brain.[7] The ameliorative mechanism involves both cholinergic and non-cholinergic pathways.[8][9]
The clinical translatable doses of phenserine show relatively high tolerability and rarely manifest severe adverse effects.[10] With respect to overdosing of the drug (20 mg/kg), a few cholinergic adverse effects were reported, including nausea and tremor which are not life-threatening.[11]
An administration form of phenserine, (-)-phenserine tartrate, which exhibits high bioavailability and solubility, is taken by mouth. Phenserine and its metabolites can readily access the brain with high permeability across the blood-brain barrier and sustain to act for a long duration with the relatively short half-life. Posiphen ((+)-phenserine), the enantiomer of (-)-phenserine, is also a potential drug by itself or synergically with (-)-phenserine, to mitigate the progression of neurological diseases, mainly Alzheimer's disease.[3]
History
Phenserine was first investigated as a substitute for physostigmine which failed to satisfy the clinical standards for treating Alzheimer's disease, and developed into more compatible remedy.[3] It was initially invented by Nigel Greig whose laboratory is affiliated with the National Institute on Aging (NIA) under the US National Institutes of Health (NIH) which subsequently released a patent of phenserine as an AChE inhibitor in 1995.[3][12] During phase I in 2000, the supplementary patent regarding its inhibitory mechanism upon β-amyloid precursor protein (APP) synthesis was added.[5] Following 6 years of phase I and II trials, Axonyx Corporation had licensed[6] phenserine to Daewoong Pharmaceutical[13] and QR Pharma (later adopted new corporation name, Annovis Bio)[14] companies in 2006, which then planned to undertake phase III trial and merchandize the drug. However, the clinical deficits ̶ representatively from a double-blinded, placebo-controlled and 7-month phase III trial which had been conducted on 377 mild to moderate Alzheimer's disease patients across Austria, Croatia, Spain, and UK[5] ̶ were discovered and no significance was exhibited for the drug efficacy. This led to the relinquishment of phenserine development,[5] merely displaying its marketable potential.
Approval status
Phenserine failed in phase III of Alzheimer's disease-aimed clinical trials and there has yet been no promise of the trial resumption since 2006.[5] The methodological problems of trials are frequently speculated as the principal reason for the failure of FDA approval as well as the scarcity of Alzheimer's disease drugs.[6] The underlying complications are generated by an inordinate variance in clinical outcomes and poor determination in optimal dosing.[5][6]
Intra and inter-site variations were incurred by a lack of baseline evaluation and longitudinal assessment on placebo groups.[6] This produced an inadequate power and, thus, appeared to have insufficient statistical significance. In light of the dose determination, the criteria for human subject engagement was not meticulously established before dosing and the effective dose range was not completely established in phase I and II, yet still persisting to phase III.[5] Compared to other Alzheimer's disease drugs, such as donepenzil, tancrine and metrifonate, the clinical advancement of phenserine involves comparably high compliance in outcome measures and protocol regimentation on methods and the clinical phase transition.[5]
Pharmacological benefits
Phenserine was invented as the Alzheimer's disease-oriented treatment in particular, and also proven to have alleviative effects upon other neurological disorders, Parkinson's disease,[9] dementia and amyotrophic lateral sclerosis.[15] The administration of phenserine within the short delay of the disease onset was shown to diminish the severity of neurodegeneration and accompanying cognitive impairments.[4] Its post-injury intervention at clinical translatable doses has been shown to significantly mitigate various neurodegenerative manifestations, preventing chronic deterioration in cognitive functions.[10] The collective neuropathological cascades in the brain are either naturally occurring or provoked by mild or moderate traumatic brain injuries,[16] such as concussion, diffuse axonal injury, ischemic and hypoxic brain injuries[8][9][10] The traumatic brain injuries have been substantially examined and induced to form test groups in phenserine research.[10][17] They are highly correlated with the onset of neurodegenerative disorders, precipitating cognitive, and behavioral impairments.
Phenserine was proven to mitigate the multiple cascades of neuropathology, triggered by traumatic brain injuries, via both cholinergic and non-cholinergic mechanisms.[8][9]
Cholinergic mechanism
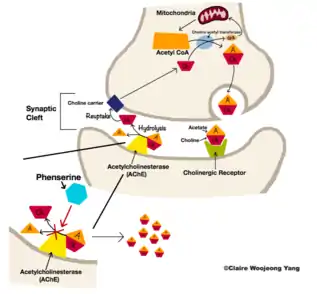
Phenserine serves as an acetylcholinesterase (AChE) inhibitor which selectively acts on the acetylcholinesterase enzyme.[3] It prevents acetylcholine from being hydrolyzed by the enzyme and enables the neurotransmitter to be further retained at the synaptic clefts.[18] Such mechanism promotes the cholinergic neuronal circuits to be activated and thereby enhances memory and cognition in Alzheimer's subjects.[17]
Non-cholinergic mechanism
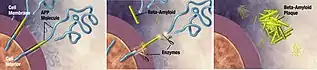
In clinical trials, phenserine was demonstrated to alleviate neurodegeneration, repressing the programmed neuronal cell death and enhancing the stem cell survival and differentiation. The alleviation can be achieved by increase in levels of neurotrophic BDNF and anti-apoptotic protein, Bcl-2, which subsequently reduces expressions of pro-apoptotic factors, GFAP and activates caspase 3.[4][8] The treatment also suppresses the levels of Alzheimer's disease-inducing proteins which are β-amyloid precursor protein (APP)[19] and Aβ peptide.[8][20] The drug interaction with the APP gene mediates the expression of both APP and its following product, Aβ protein. This regulating action reverses the glial cell-favored differentiation and increases the neuronal cell output.[17]
Phenserine also attenuates the neuroinflammation which involves the excessive activation of microglial cells to remove the cellular wastes from injury lesions. The accumulation of the activated glia near the site of brain injury is unnecessarily prolonged, stimulating the oxidative stress.[21] The inflammatory response was significantly weakened with the introduction of phenserine, which was evidenced with discouraged expression of pro-inflammatory markers, IBA 1 and TNF-α.[10] The disrupted integrity of the blood brain barrier by a degrading chemical, MMP-9, leading to neuroinflammation, is restored by phenserine as well.[8]
The alpha-synucleins, the toxic aggregates resulting from the protein misfolding, are highly observed in Parkinson's disease. The drug therapy was proven to neutralize the toxicity of alpha-synucleins via protein translation, alleviating the symptoms of the disease.[9]
Dosage
Clinically, the translatable dose of phenserine was primarily employed within a range of 1 to 5 mg/kg where the unit calibration took account of the body surface area.[4][8] This standard dose range was generally well tolerated in long term trials[10] by neuronal cell cultures, animal models and humans. Increment in dosing by 10 mg/kg is still tolerated without instigating any physiological implication.[2] The maximal administration of phenserine up to 15 mg/kg was reported in rats.[2]
Overdose
The dose of 20 mg/kg and above are appraised as overdosing in which cholinergic adverse effects ensue.
The symptoms of overdosing includes:[11]
- Nausea
- Vomiting
- Dizziness
- Tremors
- Bradycardia
Mild symptoms were notified in clinical trials but no other seriously considerable adverse effects were expressed.[5] Tremor was also noted as one of the dose-limiting actions.[2]
Chemistry
Pharmacokinetics
Oral bioavailability of phenserine was shown to be very high, up to 100%. Its bioavailability was tested by computing the drug's delivery rate across the rat's blood brain barrier.[22] The drug concentration, reached in the brain, is 10-fold higher than plasma levels,[23] verifying phenserine as a brain-permeable AChE inhibitor.[24]
Relative to its short plasma half life of 8 to 12 minutes, phenserine exhibits a long duration of action with the half-life of 8.25 hours in which the hindering effect on AChE is time-dependently faded. With the administration of phenserine, 70% or higher AChE inhibitory action in the blood was observed in preclinical studies and with systemic phenserine administration, the extracellular ACh level in the striatum increased up to three times.[24] Through PET studies and microdialysis, the compound's brain permeability was able to be further elucidated.[25]
Enantiomer (posiphen)

(-)-Phenserine, generally referred to phenserine, acts as an active enantiomer for the inhibition of acetylcholinesterase (AChE) while posiphen, its alternative enantiomer, was comparably demonstrated as a poor AChE inhibitor.[26]
In the history of posiphen research, several companies were interactively involved. In 2005, an Investigational New Drug (IND) application of posiphen was filed with FDA by TorreyPines Therapeutics while its phase I trial on animal models had been implemented by Axonyx.[27] Axonyx and TorreyPines Therapeutics officially signed for their merger agreement[27] in 2006 and licensed the drug to QR Pharma in 2008.[28] The clinical trials of posiphen against Alzheimer's disease are still underway.
Although the ongoing clinical trials yet have affirmed if posiphen can be pursued as a novel Alzheimer's disease's treatment, posiphen has demonstrated its potential by impeding neurodegenerative mechanisms. Posiphen performs in a noncholinergic manner, including but not limited to, the suppressing action on APP translation[29] and β-secretase Activity.[30][31] The β-secretase Activity is instigated by the accumulation of Aβ peptides which are augmented along with aging of the human brain.[32] In clinical demonstrations with brain cell-lines, two contrasting forms showed the noncholinergic mechanism to an equal extent, with respect to their potency and efficacy.[3][33]
Posiphen is relatively well tolerated with the administration of doses, even higher than the maximally tolerable dose of (-)-phenserine. In vivo, the levels of APP protein in the cortex were reduced by posiphen with an ED50 (median Effective Dose).[3][34] The β-secretase activity in the mouse brain also could be reduced with elevated doses of 35 and 50 mg/kg. Overall, the dose range from 10 mg to 160 mg of posiphen is well tolerated and generally adopted in clinical uses. With higher doses, supralinear increase of plasma levels was shown, indicating the saturable metabolism, which is a factor related to toxicity. Studies have shown that plasma levels of posiphen reducing brain Aβ levels are equal or greater in humans than mice.[22] Once posiphen is exceedingly dosed over 160 mg, gastro-intestinal related symptoms including nausea and vomiting, were manifested.[22] The drug additionally presents the rapid absorption rate, occurred within an hour or two. Pharmacokinetics of posiphen was overall kept linear.
Current research
A 5-year double blinded, donepezil-controlled clinical study for validation of Alzheimer's disease course modification using phenserine has been undertaken as from 2018, involving 200 patients in the UK and US. The study aims to reduce variation in AD therapeutic response between patients via optimal dose formulation.[4]
References
- Greig NH, De Micheli E, Holloway HW, Yu QS, Utsuki T, Perry TA, et al. (2000-01-02). "The experimental Alzheimer drug phenserine: preclinical pharmacokinetics and pharmacodynamics". Acta Neurologica Scandinavica. Supplementum. 176 (s176): 74–84. doi:10.1034/j.1600-0404.2000.00311.x. PMID 11261809. S2CID 25710804.
- Greig NH, Sambamurti K, Yu QS, Brossi A, Bruinsma GB, Lahiri DK (July 2005). "An overview of phenserine tartrate, a novel acetylcholinesterase inhibitor for the treatment of Alzheimer's disease". Current Alzheimer Research. 2 (3): 281–90. doi:10.2174/1567205054367829. PMID 15974893.
- Klein J (July 2007). "Phenserine". Expert Opinion on Investigational Drugs. 16 (7): 1087–97. doi:10.1517/13543784.16.7.1087. PMID 17594192. S2CID 219292296.
- Becker RE, Greig NH, Lahiri DK, Bledsoe J, Majercik S, Ballard C, et al. (2018). "(-)-Phenserine and Inhibiting Pre-Programmed Cell Death: In Pursuit of a Novel Intervention for Alzheimer's Disease". Current Alzheimer Research. 15 (9): 883–891. doi:10.2174/1567205015666180110120026. PMC 6039273. PMID 29318971.
- Becker RE, Greig NH (December 2012). "Was phenserine a failure or were investigators mislead by methods?". Current Alzheimer Research. 9 (10): 1174–81. doi:10.2174/156720512804142912. PMC 5182048. PMID 23227991.
- Becker RE, Greig NH (November 2010). "Why so few drugs for Alzheimer's disease? Are methods failing drugs?". Current Alzheimer Research. 7 (7): 642–51. doi:10.2174/156720510793499075. PMC 3010269. PMID 20704560.
- Sharma K (August 2019). "Cholinesterase inhibitors as Alzheimer's therapeutics (Review)". Molecular Medicine Reports. 20 (2): 1479–1487. doi:10.3892/mmr.2019.10374. PMC 6625431. PMID 31257471.
- Luo C, Pan SY (January 2015). "The pathways by which mild hypothermia inhibits neuronal apoptosis following ischemia/reperfusion injury". Neural Regeneration Research. 10 (1): 153–8. doi:10.4103/1673-5374.150725. PMC 4357100. PMID 25788937.
- Hsueh SC, Lecca D, Greig NH, Wang JY, Selman W, Hoffer BJ, et al. (2019-06-10). "(-)-Phenserine Ameliorates Contusion Volume, Neuroinflammation, and Behavioral Impairments Induced by Traumatic Brain Injury in Mice". Cell Transplantation. 28 (9–10): 1183–1196. doi:10.1177/0963689719854693. PMC 6767878. PMID 31177840.
- Greig NH, Lecca D, Hsueh SC, Nogueras-Ortiz C, Kapogiannis D, Tweedie D, et al. (June 2020). "(-)-Phenserine tartrate (PhenT) as a treatment for traumatic brain injury". CNS Neuroscience & Therapeutics. 26 (6): 636–649. doi:10.1111/cns.13274. PMC 7248544. PMID 31828969.
- Sugaya K (2015-04-08). "A method of biasing implanted human neural stem cells away from differentiation into glial cells by (+) phenserine to modulate the concentration of soluble ßapp in tissue or csf". Ucf Patents.
- Thatte U (July 2005). "Phenserine Axonyx". Current Opinion in Investigational Drugs. 6 (7): 729–39. PMID 16044670.
- "Axonyx Announces License of Phenserine to Daewoong Pharmaceutical Company for South Korea". www.businesswire.com. 2006-01-04. Retrieved 2020-04-06.
- "Phenserine - Next Generation AChE Inhibitor". Clinical Trials Arena. Retrieved 2020-04-06.
- Becker RE, Kapogiannis D, Greig NH (April 2018). "Does traumatic brain injury hold the key to the Alzheimer's disease puzzle?". Alzheimer's & Dementia. 14 (4): 431–443. doi:10.1016/j.jalz.2017.11.007. PMC 5958613. PMID 29245000.
- Lecca D, Bader M, Tweedie D, Hoffman AF, Jung YJ, Hsueh SC, et al. (October 2019). "(-)-Phenserine and the prevention of pre-programmed cell death and neuroinflammation in mild traumatic brain injury and Alzheimer's disease challenged mice". Neurobiology of Disease. 130: 104528. doi:10.1016/j.nbd.2019.104528. PMC 6716152. PMID 31295555.
- Hoffer BJ, Pick CG, Hoffer ME, Becker RE, Chiang YH, Greig NH (September 2017). "Repositioning drugs for traumatic brain injury - N-acetyl cysteine and Phenserine". Journal of Biomedical Science. 24 (1): 71. doi:10.1186/s12929-017-0377-1. PMC 5591517. PMID 28886718.
- Stanciu GD, Luca A, Rusu RN, Bild V, Beschea Chiriac SI, Solcan C, et al. (December 2019). "Alzheimer's Disease Pharmacotherapy in Relation to Cholinergic System Involvement". Biomolecules. 10 (1): 40. doi:10.3390/biom10010040. PMC 7022522. PMID 31888102.
- Rizvi SI, Çakatay U (2018-11-02). Molecular Basis and Emerging Strategies for Anti-aging Interventions. Springer. ISBN 978-981-13-1699-9.
- Jain KK (2011-02-14). The Handbook of Neuroprotection. Springer Science & Business Media. ISBN 978-1-61779-049-2.
- Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F (April 2018). "Oxidative stress and the amyloid beta peptide in Alzheimer's disease". Redox Biology. 14: 450–464. doi:10.1016/j.redox.2017.10.014. PMC 5680523. PMID 29080524.
- Bruinsma G, Cullen E, Greig NH, Lahiri D, Sambamurti K, Friedhoff L (2006-07-01). "P1-004: Oral treatment of mice with Posiphen™ significantly lowers brain levels of beta amyloid (1-42)". Alzheimer's & Dementia. 2 (3S_Part_4): S95. doi:10.1016/j.jalz.2006.05.379. ISSN 1552-5260. S2CID 54399849.
- Becker, Robert E, Author. (6 December 2012). Alzheimer Disease : From Molecular Biology to Therapy. ISBN 978-1-4612-4116-4. OCLC 1058877507.
{{cite book}}:|last=has generic name (help)CS1 maint: multiple names: authors list (link) - Skaddan MB, Kilbourn MR, Snyder SE, Sherman PS (February 2001). "Acetylcholinesterase inhibition increases in vivo N-(2-[18F]fluoroethyl)-4-piperidyl benzilate binding to muscarinic acetylcholine receptors". Journal of Cerebral Blood Flow and Metabolism. 21 (2): 144–8. doi:10.1097/00004647-200102000-00005. PMID 11176279. S2CID 7644319.
- Skaddan MB, Jewett DM, Sherman PS, Kilbourn MR (July 2002). "(R)-N-[11C]methyl-3-pyrrolidyl benzilate, a high-affinity reversible radioligand for PET studies of the muscarinic acetylcholine receptor". Synapse. 45 (1): 31–7. doi:10.1002/syn.10079. hdl:2027.42/34995. PMID 12112411. S2CID 9328758.
- Winblad B, Giacobini E, Frölich L, Friedhoff LT, Bruinsma G, Becker RE, Greig NH (2011-01-07). "Phenserine efficacy in Alzheimer's disease". Journal of Alzheimer's Disease. 22 (4): 1201–8. doi:10.3233/jad-2010-101311. PMC 5161452. PMID 20930279.
- "Axonyx and TorreyPines Therapeutics Announce Merger Agreement; Transaction Creates Robust Portfolio in the CNS Disease Area". www.businesswire.com. 2006-06-08. Retrieved 2020-04-08.
- "TorreyPines Therapeutics licenses Posiphen, bisnorcymcerine, phenserine to QR Pharma - Quick Facts". RTTNews. Retrieved 2020-04-08.
- Bandyopadhyay S, Rogers JT (April 2014). "Alzheimer's disease therapeutics targeted to the control of amyloid precursor protein translation: maintenance of brain iron homeostasis". Biochemical Pharmacology. Alzheimer's Disease – Amyloid, Tau and Beyond. 88 (4): 486–94. doi:10.1016/j.bcp.2014.01.032. PMC 4064675. PMID 24513321.
- Maccecchini ML, Chang MY, Pan C, John V, Zetterberg H, Greig NH (September 2012). "Posiphen as a candidate drug to lower CSF amyloid precursor protein, amyloid-β peptide and τ levels: target engagement, tolerability and pharmacokinetics in humans". Journal of Neurology, Neurosurgery, and Psychiatry. 83 (9): 894–902. doi:10.1136/jnnp-2012-302589. PMC 3415310. PMID 22791904.
- Teich AF, Sharma E, Barnwell E, Zhang H, Staniszewski A, Utsuki T, et al. (2018-01-17). "Translational inhibition of APP by Posiphen: Efficacy, pharmacodynamics, and pharmacokinetics in the APP/PS1 mouse". Alzheimer's & Dementia. 4 (1): 37–45. doi:10.1016/j.trci.2017.12.001. PMC 6021259. PMID 29955650.
- Pan X, Green BD (January 2019). "Temporal Effects of Neuron-specific beta-secretase 1 (BACE1) Knock-in on the Mouse Brain Metabolome: Implications for Alzheimer's Disease". Neuroscience. 397: 138–146. doi:10.1016/j.neuroscience.2018.11.031. PMID 30496823. S2CID 53721607.
- Lahiri DK, Chen D, Maloney B, Holloway HW, Yu QS, Utsuki T, et al. (January 2007). "The experimental Alzheimer's disease drug posiphen [(+)-phenserine] lowers amyloid-beta peptide levels in cell culture and mice". The Journal of Pharmacology and Experimental Therapeutics. 320 (1): 386–96. doi:10.1124/jpet.106.112102. PMID 17003227. S2CID 25507424.
- Chen J, Pan H, Chen C, Wu W, Iskandar K, He J, et al. (2014-06-23). "(-)-Phenserine attenuates soman-induced neuropathology". PLOS ONE. 9 (6): e99818. Bibcode:2014PLoSO...999818C. doi:10.1371/journal.pone.0099818. PMC 4067273. PMID 24955574.
- "Phenserine". www.drugbank.ca. Retrieved 2020-04-27.
- Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. (January 2018). "DrugBank 5.0: a major update to the DrugBank database for 2018". Nucleic Acids Research. 46 (D1): D1074–D1082. doi:10.1093/nar/gkx1037. PMC 5753335. PMID 29126136.