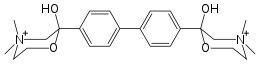
Hemicholinium-3
Hemicholinium-3 (HC3), also known as hemicholine, is a drug which blocks the reuptake of choline by the high-affinity choline transporter (ChT; encoded in humans by the gene SLC5A7) at the presynapse. The reuptake of choline is the rate-limiting step in the synthesis of acetylcholine; hence, hemicholinium-3 decreases the synthesis of acetylcholine. It is therefore classified as an indirect acetylcholine antagonist.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 2-[4-[4-(2-hydroxy-4,4-dimethylmorpholin-4-ium-2-yl)phenyl]phenyl]-4,4-dimethylmorpholin-4-ium-2-ol |
| ATC code |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.663 |
| Chemical and physical data | |
| Formula | C24H34N2O42+ |
| Molar mass | 414.546 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
Acetylcholine is synthesized from choline and a donated acetyl group from acetyl-CoA, by the action of choline acetyltransferase (ChAT). Thus, decreasing the amount of choline available to a neuron will decrease the amount of acetylcholine produced. Neurons affected by hemicholinium-3 must rely on the transport of choline from the soma (cell body), rather than relying on reuptake of choline from the synaptic cleft.
Toxicity
Hemicholinium-3 is highly toxic because it interferes with cholinergic neurotransmission. The LD50 of hemicholinium-3 for mice is about 35 μg.[2]
See also
- Triethylcholine
- Vesamicol
References
- Carlson NR (2007). Physiology of Behavior (9th ed.). Boston: Pearson Education, Inc. p. 117. ISBN 978-0-205-46724-2.
- Freeman JJ, Kosh JW, Parrish JS (October 1982). "Peripheral toxicity of hemicholinium-3 in mice". British Journal of Pharmacology. 77 (2): 239–44. doi:10.1111/j.1476-5381.1982.tb09291.x. PMC 2044599. PMID 7139185.