SNAP25
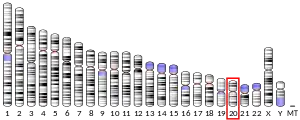
Synaptosomal-Associated Protein, 25kDa (SNAP-25) is a Target Soluble NSF (N-ethylmaleimide-sensitive factor) Attachment Protein Receptor (t-SNARE) protein encoded by the SNAP25 gene found on chromosome 20p12.2 in humans.[5][6] SNAP-25 is a component of the trans-SNARE complex, which accounts for membrane fusion specificity and directly executes fusion by forming a tight complex that brings the synaptic vesicle and plasma membranes together.[7]
Structure and function

SNAP-25, a Q-SNARE protein, is anchored to the cytosolic face of membranes via palmitoyl side chains covalently bound to cysteine amino acid residues in the central linker domain of the molecule. This means that SNAP-25 does not contain a trans-membrane domain.[9]
SNAP-25 has been identified to contribute two[10] α-helices to the SNARE complex, a four-α-helix domain complex.[11] The SNARE complex participates in vesicle fusion, which involves the docking, priming and merging of a vesicle with the cell membrane to initiate an exocytotic event. Synaptobrevin, a protein that is a part of the vesicle-associated membrane protein (VAMP) family, and syntaxin-1 also help form the SNARE complex by each contributing a single α-helix. SNAP-25 assembles with synaptobrevin and syntaxin-1, and the selective binding of these proteins enables vesicle docking and fusion to occur at active zones on the plasma membrane.[12] The energy needed for fusion to occur, results from the assembly of the SNARE proteins along with additional Sec1/Munc18-like (SM) proteins.[13]
To form the SNARE complex, synaptobrevin, syntaxin-1, and SNAP-25 associate and begin to wrap around each other to form a coiled coil quaternary structure. The α-helices of both synaptobrevin and syntaxin-1 bind to those of SNAP-25. Synaptobrevin binds the α-helix near the C-terminus of SNAP-25, while syntaxin-1 binds the α-helix near the N-terminus.[9] Dissociation of the SNARE complex is driven by ATPase N-ethylmaleimide-sensitive fusion (NSF) protein.[13]
SNAP-25 inhibits presynaptic P-, Q-, and L-type voltage-gated calcium channels[14] and interacts with the synaptotagmin C2B domain in a Ca2+-independent fashion.[15] In glutamatergic synapses, SNAP-25 decreases the Ca2+ responsiveness, while it is normally absent in GABAergic synapses.[16]
Two isoforms (mRNA splice variants) of SNAP-25 exist, which are SNAP-25a and SNAP-25b. The two isoforms differ by nine amino acid residues, including a re-localization of one of the four palmitoylated cysteine residues involved in membrane attachment.[17] The major characteristics of these two forms are outlined in the table below.
| SNAP25a | SNAP25b | |
|---|---|---|
| Structure | N-terminal α-helix
Random coil linker region with four cysteines clustered towards the center C-terminal α-helix |
N-terminal α-helix
Random coil linker region with four cysteines clustered towards the C-terminus C-terminal α-helix |
| Expression | Major SNAP-25 isoform in embryos and developing neural tissue
Minimal expression in adult tissue except in pituitary and adrenal gland tissues |
Minimal expression during development, major isoform in adult neural tissue[18] |
| Localization | Diffuse | Localized to terminals and varicosities[18] |
SNAP-25 not only plays a role in synaptogenesis and the exocytotic release of neurotransmitters, but it also affects spine morphogenesis and density, post synaptic receptor trafficking and neuronal plasticity. Other non-neuronal processes such as metabolism can also be affected by SNAP-25 protein expression.[19][20]
Clinical significance
Developmental and epileptic encephalopathies (DEEs)
Individuals harboring pathogenic heterozygous de novo missense or loss-of-function variants in SNAP-25 often present with an early-onset developmental and epileptic encephalopathy. The core symptoms comprise intellectual disability ranging between mild to profound and early-onset seizures mostly occurring before the age of two years. Further recurrent symptoms include movement disorders, cerebral visual impairment, and brain atrophy.[21] Electrophysiological studies identified aberrant spontaneous neurotransmission as causative and suggest that structurally clustered pathogenic variants lead to similar synaptic phenotypes.[22]
Attention Deficit Hyperactivity Disorder (ADHD)
Consistent with the regulation of synaptic Ca2+ responsiveness, heterozygous deletion of the SNAP-25 gene in mice results in a hyperactive phenotype similar to attention deficit hyperactivity disorder (ADHD). In heterozygous mice, a decrease in hyperactivity is observed with dextroamphetamine (or Dexedrine), an active ingredient in the ADHD drug Adderall. Homozygous deletions of the SNAP-25 gene are lethal. An additional study indicated that incorporation of a SNAP-25 transgene back into the heterozygous SNAP-25 mutant mouse can rescue normal activity levels similar to wildtype mice. This suggests that low protein levels of SNAP-25 can be a cause of hyper-kinetic behavior.[23] Subsequent studies have suggested that at least some of the SNAP-25 gene mutations in humans might predispose to ADHD.[24][25] Identification of polymorphisms in the 3’ untranslated region of the SNAP-25 gene was established in linkage studies with families that had been pre-diagnosed ADHD.[26]
Schizophrenia
Studies in the post mortem brains of patients with Schizophrenia have shown that altered protein levels of SNAP-25 are specific to regions of the brain. Reduced SNAP-25 protein expression has been observed in the hippocampus as well as an area of the frontal lobe known as Broadman’s area 10 whereas SNAP-25 expression has increased in both the cingulate cortex and prefrontal lobe of Broadman’s area 9. The varying levels of SNAP-25 protein found in different areas of the brain have been thought to contribute to the conflicting psychological behaviors (depressive vs. hyperactive) expressed in some Schizophrenic patients.[27][28][29][30]
The blind-drunk (Bdr) mouse model which has a point mutations in the SNAP-25b protein has provided a complex phenotype involving behaviors such as an abnormal circadian rhythm,[31] uncoordinated gait, and disinterest in new objects/toys.[32] Another mouse model generated from Cre-LoxP recombination, showed that conditional knockout (cKO) of the SNAP-25 gene in the forebrain, showed inactive SNAP-25 gene expression in glutamatergic neurons. However, significant glutamate levels were found in the cortex of these cKO mice.[33] These mice also exhibited deficient social skills, impaired learning and memory, enhanced kinesthetic activity, a reduced startle response, impaired self-care, nursing ability and nest-building skills. Antipsychotic drugs such as Clozapine and Riluzole have been shown to significantly reduce the schizophrenic phenotype expressed in SNAP-25 cKO mice.[33]
Alzheimer's disease
Individuals with Alzhiemer’s disease have been shown to have decreased presynaptic protein levels and impaired synaptic function in neurons. SNAP-25 can be used as a biomarker in the cerebral spinal fluid (CSF) of patients exhibiting different variations of Alzheimer's disease (prodromal Alzheimer’s and overt Alzheimer’s). Increased levels of SNAP-25 protein were observed in patients with Alzheimer’s compared to control individuals. Additionally, the presence of truncated SNAP-25 protein can be seen in the CSF of some patients with this disease. [34] In five distinct regions of the brain, low levels of SNAP-25 can be seen in patients with Alzheimer’s.[35]
Bipolar disorder
A single nucleotide polymorphism in the SNAP-25 gene promoter has been shown to influence the expression levels of the SNAP-25b isoform in the prefrontal cortex. Increased levels of SNAP-25b have been shown to impair synaptic transmission and maturation which could lead to early-onset bipolar disorder (EOBD).The most abundant isoform of SNAP-25 is SNAP-25a during the early weeks of development in mice however in adulthood there is a change and the SNAP-25b isoform increases in the brain. This is shown to correlate with adolescent humans being increasingly diagnosed with EOBD during puberty.[36] It has been suggested that early-onset bipolar disorder is more closely linked to Schizophrenia than to Bipolar Disorder itself. The single nucleotide polymorphism of SNAP-25 (rs6039769) associated with EOBD has been shown to increase the risk of patients developing Schizophrenia.[19]
Botulism
A genome wide association study pointed to the rs362584 polymorphism in the gene as possibly associated with the personality trait neuroticism.[37] Botulinum toxins A, C and E cleave SNAP-25,[38] leading to paralysis in clinically developed botulism.
Epilepsy
Deletion of the SNAP-25b isoform has been shown to cause developmental abnormalities and seizures in mice. High levels of SNAP-25a and the protein syntaxin appear to be linked to seizures found in infantile-epilepsy. SNAP-25 knock-in mice have distinct phenotypic behavior similar to the fits and seizures of epileptic patients, as well as anxiety.[39]
Learning disabilities
In the coloboma hyperactive mutant mouse model where SNAP-25 protein levels are reduced to 50% of the normal level, depolarized neurotransmitter release of dopamine and serotonin were reduced as well as glutamate release. The reduction in glutamate levels can lead to deficient memory and increased learning disabilities.[40] Certain polymorphisms of SNAP-25 (rs363043, rs353016, rs363039, rs363050) have been shown to affect the cognitive behavior, specifically the Intelligence Quotient (IQ)), of patients without pre-existing neurological diseases.[41]
Neonatal development
SNAP-25 protein expression can be altered by sex hormone levels in neonatal rats. Male rats that received an antiestrogen drug showed a 30% decrease in SNAP-25 levels and females treated with estrogen or testosterone showed a 30% increase in SNAP-25 levels.[42] This suggests that synaptosomal proteins, such as SNAP-25, may have a dependence on neonatal hormone levels during brain development in rats. An additional study, showed that SNAP-25 levels in the hippocampus of the brain in neonatal mice were altered if the mother had been exposed to human influenza virus during pregnancy.[43]
Impact in other non-humans
Loss is lethal to Drosophila, but can be fully substituted by overexpression of the related SNAP-24.[10]
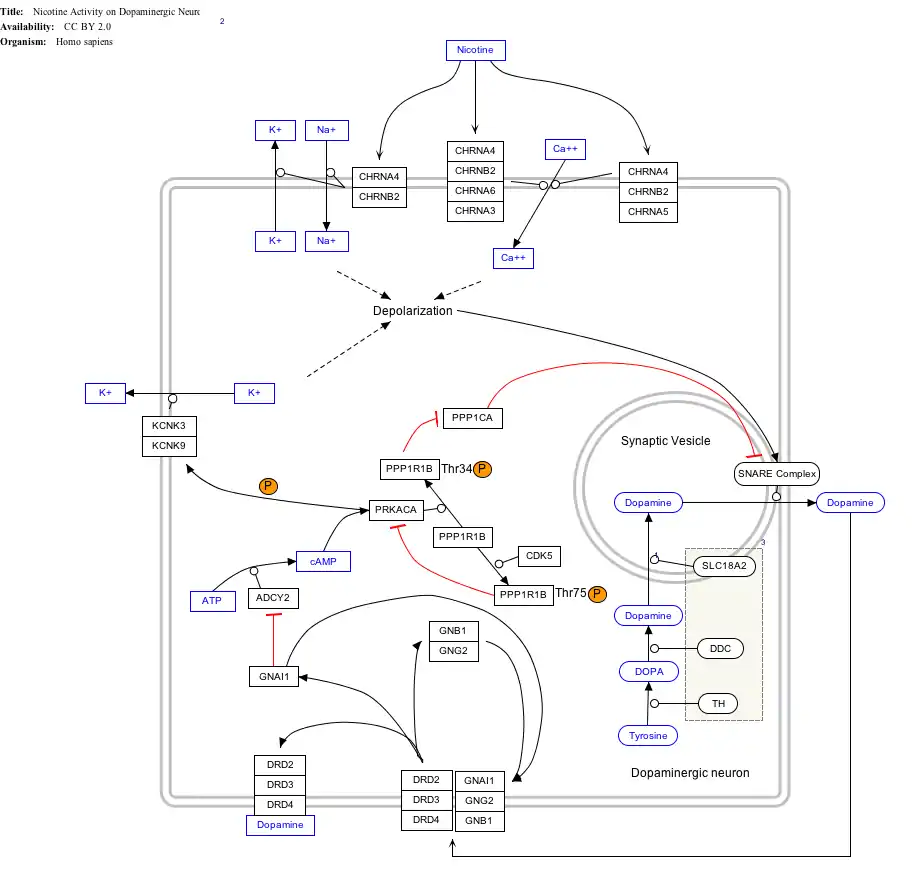
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles.[§ 1]
- The interactive pathway map can be edited at WikiPathways: "NicotineDopaminergic_WP1602".
Interactions
SNAP-25 has been shown to interact with:
- CPLX1,[44][45]
- ITSN1,[46]
- KIF5B,[47]
- SNAPAP[48] and
- STX11,[49][50]
- STX1A,[44][48][49][51][52][53][54][55][56][57][58]
- STX2,[51][52]
- STX4,[51][52][53][59]
- SYT1,[60][61]
- Syntaxin 3,[51][52][53]
- TRIM9,[57] and
- VAMP2.[44][57][62]
- Synaptotagmin binds SNAP-25 and syntaxins in the presence of Ca2+ (and thus the entire SNARE complex)[10]
References
- GRCh38: Ensembl release 89: ENSG00000132639 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000027273 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- Maglott DR, Feldblyum TV, Durkin AS, Nierman WC (May 1996). "Radiation hybrid mapping of SNAP, PCSK2, and THBD (human chromosome 20p)". Mammalian Genome. 7 (5): 400–1. doi:10.1007/s003359900120. PMID 8661740. S2CID 34951074.
- Najera K, Fagan BM, Thompson PM (November 2019). "SNAP-25 in Major Psychiatric Disorders: A Review". Neuroscience. SNARE Proteins: A Long Journey of Science in Brain Health and Disease. 420: 79–85. doi:10.1016/j.neuroscience.2019.02.008. PMID 30790667. S2CID 73486873.
- Rizo J, Südhof TC (August 2002). "Snares and Munc18 in synaptic vesicle fusion". Nature Reviews. Neuroscience. 3 (8): 641–53. doi:10.1038/nrn898. PMID 12154365. S2CID 13351502.
- Georgiev DD, Glazebrook JF (2007). "Subneuronal processing of information by solitary waves and stochastic processes". In Lyshevski SE (ed.). Nano and Molecular Electronics Handbook (PDF). Nano and Microengineering Series. CRC Press. pp. 17–1–17–41. doi:10.1201/9781315221670. ISBN 978-0-8493-8528-5.
- Chapman ER, An S, Barton N, Jahn R (November 1994). "SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils". The Journal of Biological Chemistry. 269 (44): 27427–32. doi:10.1016/S0021-9258(18)47003-2. PMID 7961655.
- Ungar D, Hughson FM (2003). "SNARE protein structure and function". Annual Review of Cell and Developmental Biology. Annual Reviews. 19 (1): 493–517. doi:10.1146/annurev.cellbio.19.110701.155609. PMID 14570579.
- Pevsner J, Hsu SC, Braun JE, Calakos N, Ting AE, Bennett MK, Scheller RH (August 1994). "Specificity and regulation of a synaptic vesicle docking complex". Neuron. 13 (2): 353–61. doi:10.1016/0896-6273(94)90352-2. PMID 8060616. S2CID 46713725.
- Calakos N, Bennett MK, Peterson KE, Scheller RH (February 1994). "Protein-protein interactions contributing to the specificity of intracellular vesicular trafficking". Science. 263 (5150): 1146–9. Bibcode:1994Sci...263.1146C. doi:10.1126/science.8108733. PMID 8108733.
- Südhof TC, Rizo J (December 2011). "Synaptic vesicle exocytosis". Cold Spring Harbor Perspectives in Biology. 3 (12): a005637. doi:10.1101/cshperspect.a005637. PMC 3225952. PMID 22026965.
- Hodel A (October 1998). "SNAP-25". The International Journal of Biochemistry & Cell Biology. 30 (10): 1069–73. doi:10.1016/S1357-2725(98)00079-X. PMID 9785471.
- Chapman ER (July 2002). "Synaptotagmin: a Ca(2+) sensor that triggers exocytosis?" (PDF). Nature Reviews. Molecular Cell Biology. 3 (7): 498–508. doi:10.1038/nrm855. PMID 12094216. S2CID 12384262. Archived from the original (PDF) on August 29, 2006.
- Verderio C, Pozzi D, Pravettoni E, Inverardi F, Schenk U, Coco S, et al. (February 2004). "SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization". Neuron. 41 (4): 599–610. doi:10.1016/S0896-6273(04)00077-7. PMID 14980208. S2CID 16171280.
- Nagy G, Milosevic I, Fasshauer D, Müller EM, de Groot BL, Lang T, et al. (December 2005). "Alternative splicing of SNAP-25 regulates secretion through nonconservative substitutions in the SNARE domain". Molecular Biology of the Cell. 16 (12): 5675–85. doi:10.1091/mbc.E05-07-0595. PMC 1289412. PMID 16195346.
- Bark IC, Hahn KM, Ryabinin AE, Wilson MC (February 1995). "Differential expression of SNAP-25 protein isoforms during divergent vesicle fusion events of neural development". Proceedings of the National Academy of Sciences of the United States of America. 92 (5): 1510–4. Bibcode:1995PNAS...92.1510B. doi:10.1073/pnas.92.5.1510. PMC 42549. PMID 7878010.
- Houenou J, Boisgontier J, Henrion A, d'Albis MA, Dumaine A, Linke J, et al. (October 2017). "SNAP25 At-Risk Variant for Bipolar Disorder and Schizophrenia". The Journal of Neuroscience. 37 (43): 10389–10397. doi:10.1523/JNEUROSCI.1040-17.2017. PMC 6596626. PMID 28972123.
- Antonucci F, Corradini I, Fossati G, Tomasoni R, Menna E, Matteoli M (2016). "SNAP-25, a Known Presynaptic Protein with Emerging Postsynaptic Functions". Frontiers in Synaptic Neuroscience. 8: 7. doi:10.3389/fnsyn.2016.00007. PMC 4805587. PMID 27047369.
- Klöckner C, Sticht H, Zacher P, Popp B, Babcock HE, Bakker DP, et al. (April 2021). "De novo variants in SNAP25 cause an early-onset developmental and epileptic encephalopathy". Genetics in Medicine. 23 (4): 653–660. doi:10.1038/s41436-020-01020-w. PMID 33299146. S2CID 228087433.
- Alten B, Zhou Q, Shin OH, Esquivies L, Lin PY, White KI, et al. (January 2021). "Role of Aberrant Spontaneous Neurotransmission in SNAP25-Associated Encephalopathies". Neuron. 109 (1): 59–72.e5. doi:10.1016/j.neuron.2020.10.012. PMC 7790958. PMID 33147442.
- Steffensen SC, Henriksen SJ, Wilson MC (November 1999). "Transgenic rescue of SNAP-25 restores dopamine-modulated synaptic transmission in the coloboma mutant". Brain Research. 847 (2): 186–95. doi:10.1016/S0006-8993(99)02023-5. PMID 10575087. S2CID 41368865.
- Brophy K, Hawi Z, Kirley A, Fitzgerald M, Gill M (2002). "Synaptosomal-associated protein 25 (SNAP-25) and attention deficit hyperactivity disorder (ADHD): evidence of linkage and association in the Irish population". Molecular Psychiatry. 7 (8): 913–7. doi:10.1038/sj.mp.4001092. hdl:2262/36350. PMID 12232787.
- Mill J, Curran S, Kent L, Gould A, Huckett L, Richards S, et al. (April 2002). "Association study of a SNAP-25 microsatellite and attention deficit hyperactivity disorder". American Journal of Medical Genetics. 114 (3): 269–71. doi:10.1002/ajmg.10253. PMID 11920846.
- Barr CL, Feng Y, Wigg K, Bloom S, Roberts W, Malone M, et al. (July 2000). "Identification of DNA variants in the SNAP-25 gene and linkage study of these polymorphisms and attention-deficit hyperactivity disorder". Molecular Psychiatry. 5 (4): 405–9. doi:10.1038/sj.mp.4000733. PMID 10889551. S2CID 22779309.
- Corradini I, Verderio C, Sala M, Wilson MC, Matteoli M (January 2009). "SNAP-25 in neuropsychiatric disorders". Annals of the New York Academy of Sciences. 1152 (1): 93–9. Bibcode:2009NYASA1152...93C. doi:10.1111/j.1749-6632.2008.03995.x. PMC 2706123. PMID 19161380.
- Gabriel SM, Haroutunian V, Powchik P, Honer WG, Davidson M, Davies P, Davis KL (June 1997). "Increased concentrations of presynaptic proteins in the cingulate cortex of subjects with schizophrenia". Archives of General Psychiatry. 54 (6): 559–66. doi:10.1001/archpsyc.1997.01830180077010. PMID 9193197.
- Thompson PM, Sower AC, Perrone-Bizzozero NI (February 1998). "Altered levels of the synaptosomal associated protein SNAP-25 in schizophrenia". Biological Psychiatry. 43 (4): 239–43. doi:10.1016/S0006-3223(97)00204-7. PMID 9513732. S2CID 20347660.
- Thompson PM, Egbufoama S, Vawter MP (May 2003). "SNAP-25 reduction in the hippocampus of patients with schizophrenia". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 27 (3): 411–7. doi:10.1016/S0278-5846(03)00027-7. PMID 12691775. S2CID 1051797.
- Oliver PL, Sobczyk MV, Maywood ES, Edwards B, Lee S, Livieratos A, et al. (February 2012). "Disrupted circadian rhythms in a mouse model of schizophrenia". Current Biology. 22 (4): 314–9. doi:10.1016/j.cub.2011.12.051. PMC 3356578. PMID 22264613.
- Jeans AF, Oliver PL, Johnson R, Capogna M, Vikman J, Molnár Z, et al. (February 2007). "A dominant mutation in Snap25 causes impaired vesicle trafficking, sensorimotor gating, and ataxia in the blind-drunk mouse". Proceedings of the National Academy of Sciences of the United States of America. 104 (7): 2431–6. Bibcode:2007PNAS..104.2431J. doi:10.1073/pnas.0610222104. PMC 1793901. PMID 17283335.
- Yang H, Zhang M, Shi J, Zhou Y, Wan Z, Wang Y, et al. (2017). "Brain-Specific SNAP-25 Deletion Leads to Elevated Extracellular Glutamate Level and Schizophrenia-Like Behavior in Mice". Neural Plasticity. 2017: 4526417. doi:10.1155/2017/4526417. PMC 5727794. PMID 29318050.
- Brinkmalm A, Brinkmalm G, Honer WG, Frölich L, Hausner L, Minthon L, et al. (November 2014). "SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer's disease". Molecular Neurodegeneration. 9: 53. doi:10.1186/1750-1326-9-53. PMC 4253625. PMID 25418885.
- Greber S, Lubec G, Cairns N, Fountoulakis M (1999). "Decreased levels of synaptosomal associated protein 25 in the brain of patients with Down syndrome and Alzheimer's disease". Electrophoresis. 20 (4–5): 928–34. doi:10.1002/(SICI)1522-2683(19990101)20:4/5<928::AID-ELPS928>3.0.CO;2-Z. PMID 10344268. S2CID 22531212.
- Etain B, Dumaine A, Mathieu F, Chevalier F, Henry C, Kahn JP, et al. (July 2010). "A SNAP25 promoter variant is associated with early-onset bipolar disorder and a high expression level in brain". Molecular Psychiatry. 15 (7): 748–55. doi:10.1038/mp.2008.148. PMC 2937032. PMID 19125158.
- Terracciano A, Sanna S, Uda M, Deiana B, Usala G, Busonero F, et al. (June 2010). "Genome-wide association scan for five major dimensions of personality". Molecular Psychiatry. 15 (6): 647–56. doi:10.1038/mp.2008.113. PMC 2874623. PMID 18957941.
- Aoki KR, Guyer B (November 2001). "Botulinum toxin type A and other botulinum toxin serotypes: a comparative review of biochemical and pharmacological actions". European Journal of Neurology. 8 Suppl 5: 21–9. doi:10.1046/j.1468-1331.2001.00035.x. PMID 11851731. S2CID 36829902.
- Rohena L, Neidich J, Truitt Cho M, Gonzalez KD, Tang S, Devinsky O, Chung WK (2013). "Mutation in SNAP25 as a novel genetic cause of epilepsy and intellectual disability". Rare Diseases. 1 (1): e26314. doi:10.4161/rdis.26314. PMC 3932847. PMID 25003006.
- Raber J, Mehta PP, Kreifeldt M, Parsons LH, Weiss F, Bloom FE, Wilson MC (January 1997). "Coloboma hyperactive mutant mice exhibit regional and transmitter-specific deficits in neurotransmission". Journal of Neurochemistry. 68 (1): 176–86. doi:10.1046/j.1471-4159.1997.68010176.x. PMID 8978724. S2CID 25505619.
- Gosso MF, de Geus EJ, van Belzen MJ, Polderman TJ, Heutink P, Boomsma DI, Posthuma D (September 2006). "The SNAP-25 gene is associated with cognitive ability: evidence from a family-based study in two independent Dutch cohorts". Molecular Psychiatry. 11 (9): 878–86. doi:10.1038/sj.mp.4001868. PMID 16801949. S2CID 437158.
- Lustig RH, Hua P, Wilson MC, Federoff HJ (October 1993). "Ontogeny, sex dimorphism, and neonatal sex hormone determination of synapse-associated messenger RNAs in rat brain". Brain Research. Molecular Brain Research. 20 (1–2): 101–10. doi:10.1016/0169-328X(93)90114-5. PMID 8255171.
- Fatemi SH, Sidwell R, Kist D, Akhter P, Meltzer HY, Bailey K, et al. (July 1998). "Differential expression of synaptosome-associated protein 25 kDa [SNAP-25] in hippocampi of neonatal mice following exposure to human influenza virus in utero". Brain Research. 800 (1): 1–9. doi:10.1016/S0006-8993(98)00450-8. PMID 9685568. S2CID 36917316.
- Chen X, Tomchick DR, Kovrigin E, Araç D, Machius M, Südhof TC, Rizo J (January 2002). "Three-dimensional structure of the complexin/SNARE complex". Neuron. 33 (3): 397–409. doi:10.1016/s0896-6273(02)00583-4. PMID 11832227. S2CID 17878965.
- Hu K, Carroll J, Rickman C, Davletov B (November 2002). "Action of complexin on SNARE complex". The Journal of Biological Chemistry. 277 (44): 41652–6. doi:10.1074/jbc.M205044200. PMID 12200427.
- Okamoto M, Schoch S, Südhof TC (June 1999). "EHSH1/intersectin, a protein that contains EH and SH3 domains and binds to dynamin and SNAP-25. A protein connection between exocytosis and endocytosis?". The Journal of Biological Chemistry. 274 (26): 18446–54. doi:10.1074/jbc.274.26.18446. PMID 10373452.
- Diefenbach RJ, Diefenbach E, Douglas MW, Cunningham AL (December 2002). "The heavy chain of conventional kinesin interacts with the SNARE proteins SNAP25 and SNAP23". Biochemistry. 41 (50): 14906–15. doi:10.1021/bi026417u. PMID 12475239.
- Ilardi JM, Mochida S, Sheng ZH (February 1999). "Snapin: a SNARE-associated protein implicated in synaptic transmission". Nature Neuroscience. 2 (2): 119–24. doi:10.1038/5673. PMID 10195194. S2CID 25524692.
- Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, et al. (September 2005). "A human protein-protein interaction network: a resource for annotating the proteome". Cell. 122 (6): 957–68. doi:10.1016/j.cell.2005.08.029. hdl:11858/00-001M-0000-0010-8592-0. PMID 16169070. S2CID 8235923.
- Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, et al. (October 2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514. S2CID 4427026.
- Hata Y, Südhof TC (June 1995). "A novel ubiquitous form of Munc-18 interacts with multiple syntaxins. Use of the yeast two-hybrid system to study interactions between proteins involved in membrane traffic". The Journal of Biological Chemistry. 270 (22): 13022–8. doi:10.1074/jbc.270.22.13022. PMID 7768895.
- Ravichandran V, Chawla A, Roche PA (June 1996). "Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues". The Journal of Biological Chemistry. 271 (23): 13300–3. doi:10.1074/jbc.271.23.13300. PMID 8663154.
- Steegmaier M, Yang B, Yoo JS, Huang B, Shen M, Yu S, et al. (December 1998). "Three novel proteins of the syntaxin/SNAP-25 family". The Journal of Biological Chemistry. 273 (51): 34171–9. doi:10.1074/jbc.273.51.34171. PMID 9852078.
- Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Südhof TC, Rizo J (August 1999). "A conformational switch in syntaxin during exocytosis: role of munc18". The EMBO Journal. 18 (16): 4372–82. doi:10.1093/emboj/18.16.4372. PMC 1171512. PMID 10449403.
- McMahon HT, Missler M, Li C, Südhof TC (October 1995). "Complexins: cytosolic proteins that regulate SNAP receptor function". Cell. 83 (1): 111–9. doi:10.1016/0092-8674(95)90239-2. PMID 7553862. S2CID 675343.
- Gonelle-Gispert C, Molinete M, Halban PA, Sadoul K (September 2000). "Membrane localization and biological activity of SNAP-25 cysteine mutants in insulin-secreting cells". Journal of Cell Science. 113 ( Pt 18) (18): 3197–205. doi:10.1242/jcs.113.18.3197. PMID 10954418.
- Li Y, Chin LS, Weigel C, Li L (November 2001). "Spring, a novel RING finger protein that regulates synaptic vesicle exocytosis". The Journal of Biological Chemistry. 276 (44): 40824–33. doi:10.1074/jbc.M106141200. PMID 11524423.
- Chapman ER, An S, Barton N, Jahn R (November 1994). "SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils". The Journal of Biological Chemistry. 269 (44): 27427–32. doi:10.1016/S0021-9258(18)47003-2. PMID 7961655.
- Reed GL, Houng AK, Fitzgerald ML (April 1999). "Human platelets contain SNARE proteins and a Sec1p homologue that interacts with syntaxin 4 and is phosphorylated after thrombin activation: implications for platelet secretion". Blood. 93 (8): 2617–26. doi:10.1182/blood.V93.8.2617. PMID 10194441.
- Gerona RR, Larsen EC, Kowalchyk JA, Martin TF (March 2000). "The C terminus of SNAP25 is essential for Ca(2+)-dependent binding of synaptotagmin to SNARE complexes". The Journal of Biological Chemistry. 275 (9): 6328–36. doi:10.1074/jbc.275.9.6328. PMID 10692432.
- Zhang X, Kim-Miller MJ, Fukuda M, Kowalchyk JA, Martin TF (May 2002). "Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis". Neuron. 34 (4): 599–611. doi:10.1016/s0896-6273(02)00671-2. PMID 12062043. S2CID 16768299.
- Hao JC, Salem N, Peng XR, Kelly RB, Bennett MK (March 1997). "Effect of mutations in vesicle-associated membrane protein (VAMP) on the assembly of multimeric protein complexes". The Journal of Neuroscience. 17 (5): 1596–603. doi:10.1523/JNEUROSCI.17-05-01596.1997. PMC 6573372. PMID 9030619.
Further reading
- Hanson PI, Otto H, Barton N, Jahn R (July 1995). "The N-ethylmaleimide-sensitive fusion protein and alpha-SNAP induce a conformational change in syntaxin". The Journal of Biological Chemistry. 270 (28): 16955–61. doi:10.1074/jbc.270.28.16955. PMID 7622514.
- Hata Y, Südhof TC (June 1995). "A novel ubiquitous form of Munc-18 interacts with multiple syntaxins. Use of the yeast two-hybrid system to study interactions between proteins involved in membrane traffic". The Journal of Biological Chemistry. 270 (22): 13022–8. doi:10.1074/jbc.270.22.13022. PMID 7768895.
- Chapman ER, An S, Barton N, Jahn R (November 1994). "SNAP-25, a t-SNARE which binds to both syntaxin and synaptobrevin via domains that may form coiled coils". The Journal of Biological Chemistry. 269 (44): 27427–32. doi:10.1016/S0021-9258(18)47003-2. PMID 7961655.
- Zhao N, Hashida H, Takahashi N, Sakaki Y (August 1994). "Cloning and sequence analysis of the human SNAP25 cDNA". Gene. 145 (2): 313–4. doi:10.1016/0378-1119(94)90027-2. PMID 8056350.
- Bark IC, Wilson MC (February 1994). "Human cDNA clones encoding two different isoforms of the nerve terminal protein SNAP-25". Gene. 139 (2): 291–2. doi:10.1016/0378-1119(94)90773-0. PMID 8112622.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Maglott DR, Feldblyum TV, Durkin AS, Nierman WC (May 1996). "Radiation hybrid mapping of SNAP, PCSK2, and THBD (human chromosome 20p)". Mammalian Genome. 7 (5): 400–1. doi:10.1007/s003359900120. PMID 8661740. S2CID 34951074.
- Ravichandran V, Chawla A, Roche PA (June 1996). "Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues". The Journal of Biological Chemistry. 271 (23): 13300–3. doi:10.1074/jbc.271.23.13300. PMID 8663154.
- Rettig J, Sheng ZH, Kim DK, Hodson CD, Snutch TP, Catterall WA (July 1996). "Isoform-specific interaction of the alpha1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25". Proceedings of the National Academy of Sciences of the United States of America. 93 (14): 7363–8. Bibcode:1996PNAS...93.7363R. doi:10.1073/pnas.93.14.7363. PMC 38990. PMID 8692999.
- Jagadish MN, Fernandez CS, Hewish DR, Macaulay SL, Gough KH, Grusovin J, et al. (August 1996). "Insulin-responsive tissues contain the core complex protein SNAP-25 (synaptosomal-associated protein 25) A and B isoforms in addition to syntaxin 4 and synaptobrevins 1 and 2". The Biochemical Journal. 317 ( Pt 3) (3): 945–54. doi:10.1042/bj3170945. PMC 1217577. PMID 8760387.
- Betz A, Okamoto M, Benseler F, Brose N (January 1997). "Direct interaction of the rat unc-13 homologue Munc13-1 with the N terminus of syntaxin". The Journal of Biological Chemistry. 272 (4): 2520–6. doi:10.1074/jbc.272.4.2520. PMID 8999968.
- Araki S, Tamori Y, Kawanishi M, Shinoda H, Masugi J, Mori H, et al. (May 1997). "Inhibition of the binding of SNAP-23 to syntaxin 4 by Munc18c". Biochemical and Biophysical Research Communications. 234 (1): 257–62. doi:10.1006/bbrc.1997.6560. PMID 9168999.
- Lane SR, Liu Y (November 1997). "Characterization of the palmitoylation domain of SNAP-25". Journal of Neurochemistry. 69 (5): 1864–9. doi:10.1046/j.1471-4159.1997.69051864.x. PMID 9349529. S2CID 6343703.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Okamoto M, Südhof TC (December 1997). "Mints, Munc18-interacting proteins in synaptic vesicle exocytosis". The Journal of Biological Chemistry. 272 (50): 31459–64. doi:10.1074/jbc.272.50.31459. PMID 9395480.
- Low SH, Roche PA, Anderson HA, van Ijzendoorn SC, Zhang M, Mostov KE, Weimbs T (February 1998). "Targeting of SNAP-23 and SNAP-25 in polarized epithelial cells". The Journal of Biological Chemistry. 273 (6): 3422–30. doi:10.1074/jbc.273.6.3422. PMID 9452464.
- Poirier MA, Hao JC, Malkus PN, Chan C, Moore MF, King DS, Bennett MK (May 1998). "Protease resistance of syntaxin.SNAP-25.VAMP complexes. Implications for assembly and structure". The Journal of Biological Chemistry. 273 (18): 11370–7. doi:10.1074/jbc.273.18.11370. PMID 9556632.
- Prekeris R, Klumperman J, Chen YA, Scheller RH (November 1998). "Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes". The Journal of Cell Biology. 143 (4): 957–71. doi:10.1083/jcb.143.4.957. PMC 2132958. PMID 9817754.
- Gonelle-Gispert C, Halban PA, Niemann H, Palmer M, Catsicas S, Sadoul K (April 1999). "SNAP-25a and -25b isoforms are both expressed in insulin-secreting cells and can function in insulin secretion". The Biochemical Journal. 339 ( Pt 1) (1): 159–65. doi:10.1042/0264-6021:3390159. PMC 1220140. PMID 10085240.
- Ilardi JM, Mochida S, Sheng ZH (February 1999). "Snapin: a SNARE-associated protein implicated in synaptic transmission". Nature Neuroscience. 2 (2): 119–24. doi:10.1038/5673. PMID 10195194. S2CID 25524692.
External links
- SNAP25+Protein at the US National Library of Medicine Medical Subject Headings (MeSH)