n-Butylamine
n-Butylamine is an organic compound (specifically, an amine) with the formula CH3(CH2)3NH2. This colourless liquid is one of the four isomeric amines of butane, the others being sec-butylamine, tert-butylamine, and isobutylamine. It is a liquid having the fishy, ammonia-like odor common to amines. The liquid acquires a yellow color upon storage in air. It is soluble in all organic solvents. Vapors heavier than air and it produces toxic oxides of nitrogen during combustion.[3]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Butan-1-amine | |
Other names
| |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| Abbreviations | NBA |
Beilstein Reference |
605269 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.364 |
| EC Number |
|
Gmelin Reference |
1784 |
| MeSH | n-butylamine |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 1125 |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C4H11N |
| Molar mass | 73.139 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | fishy, ammoniacal |
| Density | 740 mg ml−1 |
| Melting point | −49 °C; −56 °F; 224 K |
| Boiling point | 77 to 79 °C; 170 to 174 °F; 350 to 352 K |
Solubility in water |
Miscible |
| log P | 1.056 |
| Vapor pressure | 9.1 kPa (at 20 °C) |
Henry's law constant (kH) |
570 μmol Pa−1 kg−1 |
| Basicity (pKb) | 3.22 |
Magnetic susceptibility (χ) |
-58.9·10−6 cm3/mol |
Refractive index (nD) |
1.401 |
| Viscosity | 500 µPa s (at 20 °C) |
| Thermochemistry | |
Heat capacity (C) |
188 J K−1 mol−1 |
Std enthalpy of formation (ΔfH⦵298) |
−128.9–−126.5 kJ mol−1 |
Std enthalpy of combustion (ΔcH⦵298) |
−3.0196–−3.0174 MJ mol−1 |
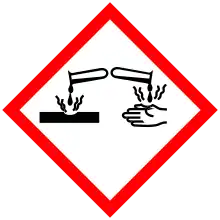
| Hazards | |
| GHS labelling: | |
Pictograms |
   |
Signal word |
Danger |
Hazard statements |
H225, H302, H312, H314, H332 |
Precautionary statements |
P210, P280, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | |
| Flash point | −7 °C (19 °F; 266 K) |
Autoignition temperature |
312 °C (594 °F; 585 K) |
| Explosive limits | 1.7–9.8% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
|
LCLo (lowest published) |
4000 ppm (rat, 4 hr) 263 ppm (mouse, 2 hr)[1] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
C 5 ppm (15 mg/m3) [skin][2] |
REL (Recommended) |
C 5 ppm (15 mg/m3) [skin][2] |
IDLH (Immediate danger) |
300 ppm[2] |
| Safety data sheet (SDS) | hazard.com |
| Related compounds | |
Related alkanamines |
|
Related compounds |
2-Methyl-2-nitrosopropane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Synthesis and reactions
It is produced by the reaction of ammonia and alcohols over alumina:
- CH3(CH2)3OH + NH3 → CH3(CH2)3NH2 + H2O
n-Butylamine is a weak base. The pKa of [CH3(CH2)3NH3]+ is 10.78.[4]
n-Butylamine exhibits reactions typical of other simple alkyl amines, i.e., alkylation, acylation, condensation with carbonyls. It forms complexes with metal ions, examples being cis- and trans-[PtI2(NH2Bu)2].[5]
Uses
This compound is used as an ingredient in the manufacture of pesticides (such as thiocarbazides), pharmaceuticals, and emulsifiers. It is also a precursor for the manufacture of N,N′-dibutylthiourea, a rubber vulcanization accelerator, and n-butylbenzenesulfonamide, a plasticizer of nylon. It is used in the synthesis of fengabine, the fungicide benomyl, and butamoxane, and the antidiabetic tolbutamide.[6]

Safety
The LD50 to rats through the oral exposure route is 366 mg/kg.[7]
In regards to occupational exposures to n-butylamine, the Occupational Safety and Health Administration and National Institute for Occupational Safety and Health have set occupational exposure limits at a ceiling of 5 ppm (15 mg/m3) for dermal exposure.[8]
References
- "N-Butylamine". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- NIOSH Pocket Guide to Chemical Hazards. "#0079". National Institute for Occupational Safety and Health (NIOSH).
- PubChem. "Butylamine". pubchem.ncbi.nlm.nih.gov. Retrieved 2022-02-15.
- H. K. Hall, Jr. (1957). "Correlation of the Base Strengths of Amines". J. Am. Chem. Soc. 79 (20): 5441–5444. doi:10.1021/ja01577a030.
- Rochon, Fernande D.; Buculei, Viorel (2004). "Multinuclear NMR Study and Crystal Structures of Complexes of the Types cis- and trans-Pt(amine)2I2". Inorganica Chimica Acta. 357 (8): 2218–2230. doi:10.1016/j.ica.2003.10.039.
- Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke, "Amines, Aliphatic" Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005.doi:10.1002/14356007.a02_001
- "n-Butylamine MSDS" (PDF). Archived from the original (PDF) on 2013-11-12. Retrieved 2013-11-12.
- CDC - NIOSH Pocket Guide to Chemical Hazards
External links
 Media related to N-butylamine at Wikimedia Commons
Media related to N-butylamine at Wikimedia Commons
