Palisade (pathology)
In histopathology, a palisade is a single layer of relatively long cells, arranged loosely perpendicular to a surface and parallel to each other.[1] A rosette is a palisade in a halo or spoke-and-wheel arrangement, surrounding a central core or hub.[2] A pseudorosette is a perivascular radial arrangement of neoplastic cells around a small blood vessel.[2]
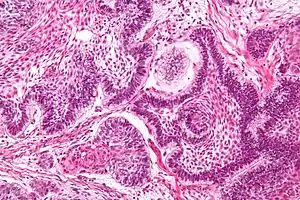
Rosette
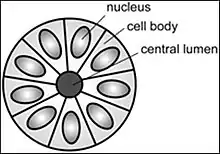
A rosette is a cell formation in a halo or spoke-and-wheel arrangement, surrounding a central core or hub. The central hub may consist of an empty-appearing lumen or a space filled with cytoplasmic processes. The cytoplasm of each of the cells in the rosette is often wedge-shaped with the apex directed toward the central core: the nuclei of the cells participating in the rosette are peripherally positioned and form a ring or halo around the hub.[2]
Pathogenesis
Rosettes may be considered primary or secondary manifestations of tumor architecture. Primary rosettes form as a characteristic growth pattern of a given tumor type whereas secondary rosettes result from the influence of external factors on tumor growth. For example, in the latter instance, regressive cell swelling may centripetally displace the cytoplasm as the nucleus is squeezed to the periphery. Although the presence of primary rosettes may suggest a given diagnosis, usually this finding alone is not considered absolutely pathognomic for one specific tumor type.[2]
Loss or gain of genetic information is the main cause of rosette and pseudorosette formation. The cell populations exhibiting neuronal differentiation are believed to secrete surface glycoproteins and glycolipids which mediate cell-to-cell recognition and adhesion. One hypothesis is that these sticky cell surface markers cause the developing cell bodies to cluster or aggregate and their primitive neurites to tangle. As the cells grow, the neurite tangle remains centrally located and the cell bodies are squeezed to the periphery, thus explaining the rosette pattern. Depending upon their location, ependymal cells may display 2 cell poles. A luminal pole projects to the ependymal lining of a ventricle and a “submesenchymal pole” projects toward the surface of the brain demonstrating glial processes and peripherally situated footplates. Frieda and Pollak conceptualize the architecture of ependymomas as a primitive neural tube turned inside out with the submesenchymal poles converging toward a central vessel, thus forming a pseudorosette rather than projecting centrifugally toward the pia.[2]
Causes
True rosettes are mainly found in neuropathologic disorder and are also present in osteosarcoma, non-Hodgkin lymphoma, fibromyxoid sarcoma, medullary thyroid carcinoma, embryonal tumor with abundant neuropil and true rosettes (ETANTR), rhambdomyosarcoma, chronic cholestasis and chronic active hepatitis, tobacco rosette: complex viral disease, malaria, adenocarcinoma in colon and rectum in the Aghamiri population, hyalinizing spindle cell fused with giant rosette, endometrial stromal sarcoma with hyalinizing giant rosettes, embryonal tumor etc.[2]
Flexner–Wintersteiner rosettes (spoke-and-wheel shaped cell formation seen mainly in retinoblastoma[3]) have been described as a form of palisading.[4]
Flexner–Wintersteiner rosette
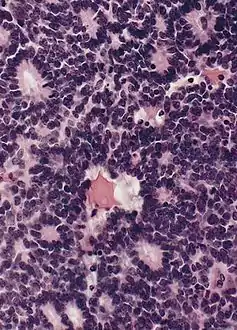
A Flexner–Wintersteiner rosette is a spoke-and-wheel shaped cell formation seen in retinoblastoma and certain other ophthalmic tumors.[3] A rosette is a structure or formation resembling a rose, such as the clusters of polymorphonuclear leukocytes around a globule of lipid nuclear material, as observed in the test for disseminated lupus erythematosus.[3]
Unlike the center of the Homer Wright rosette, the central lumen is devoid of fiber-rich neuropil. Like the Homer Wright rosette, the Flexner–Wintersteiner rosette represents a specific form of tumor differentiation.[5][6][7][8] Electron microscopy reveals that the tumor cells forming the Flexner–Wintersteiner rosette have ultrastructural features of primitive photoreceptor cells.[9] Furthermore, the rosette lumen shows similar staining patterns as in rods and cones,[10] suggesting that Flexner–Wintersteiner rosettes represent a specific form of retinal differentiation. In addition to being a characteristic finding in retinoblastomas, Flexner–Wintersteiner rosettes may also be found in pinealoblastomas and medulloepitheliomas.[5]
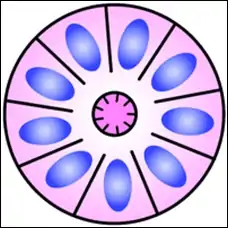 Structure of a Flexner–Wintersteiner rosette
Structure of a Flexner–Wintersteiner rosette
Flexner–Wintersteiner rosettes were first described by Simon Flexner (1863–1946), a physician, scientist, administrator, and professor of experimental pathology at the University of Pennsylvania (1899–1903). Flexner noted characteristic clusters of cells in an infantile eye tumor which he called retinoepithelioma.[11][12][13] A few years later, in 1897, Austrian ophthalmologist Hugo Wintersteiner (1865–1946) confirmed Flexner's observations and noted that the cell clusters resembled rods and cones.[14] These characteristic rosette formations were subsequently recognized as important features of retinoblastomas.
Pseudorosette
A pseudorosette is a perivascular radial arrangement of neoplastic cells around a small blood vessel. Pseudorosettes are present in neuroblastoma, medulloblastoma, malignant melanoma, ependymoma, Merkel cell carcinoma, neuroendocrine tumor of skin, seborrheic keratosis, dendritic cell neurofibroma, astroblastoma, large cell neuroendocrine tumor of cervix, clear cell ependymoma of spinal cord, celiac disease, nasal tumor of olfactory origin, rosette forming glioneural tumor (RGNT), oncocytoma, Wilm's tumor, pheochromocytoma of urinary bladder.[2]
Homer Wright pseudorosette
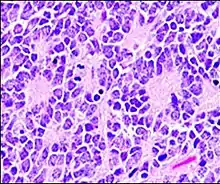
A Homer Wright pseudorosette is a type of pseudorosette in which differentiated tumor cells surround the neuropil.[15] Examples of tumors containing these are neuroblastoma, medulloblastoma, pinealoblastoma, and primitive neuroectodermal tumors of bone. Homer Wright rosettes are considered "pseudo" in the sense that they are not true rosettes. True rosettes are Flexner–Wintersteiner rosette, which contain an empty lumen. Homer Wright rosettes contain abundant fibrillary material. They are named for James Homer Wright.
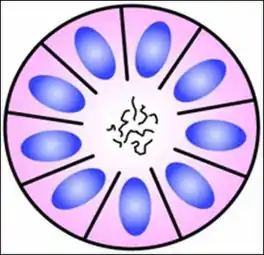 Structure of a Homer Wright pseudorosette
Structure of a Homer Wright pseudorosette
Perivascular pseudorosette
A perivascular pseudorosette consists of a spoke-wheel arrangement of cells with tapered cellular processes radiates around a wall of a centrally placed vessel. The modifier “pseudo” differentiates this pattern from the Homer Wright and Flexner-Wintersteiner rosettes, perhaps because the central structure is not actually formed by the tumor itself, but instead represents a native, non-neoplastic element. Also, some early investigators argued about the definition of a central lumen, choosing “pseudo” to indicate that the hub was not a true lumen but contained structures. Nevertheless, this pattern remains extremely diagnostically useful and the modifier unnecessarily leads to confusion. Perivascular pseudorosettes are encountered in most ependymomas regardless of grade or variant. As such, they are significantly more sensitive for the diagnosis of ependymomas than true ependymal rosettes. Unfortunately, perivascular pseudorosettes are also less specific in that they are also encountered in medulloblastomas, PNETs, central neurocytomas, and less often in glioblastomas, and a rare pediatric tumor, monomorphous pilomyxoid astrocytomas.[2]
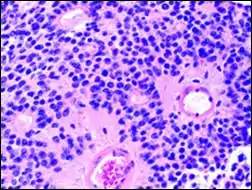 Micrograph of perivascular pseudorosettes
Micrograph of perivascular pseudorosettes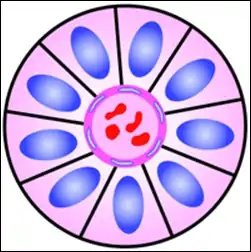 Structure of a perivascular pseudorosette
Structure of a perivascular pseudorosette
Pineocytomatous/neurocytic pseudorosettes
Histologic features of these two tumors are virtually identical, including their tendency to form neuropilrich rosettes, referred to as pineocytomatous/neurocytic rosettes in central neurocytoma. Both are quite similar to the Homer Wright rosette, but they are generally larger and more irregular in contour. The cells of the pineocytomatous/neurocytic rosettes are also considered to be much more differentiated than the cells forming Homer Wright rosettes in that the nuclei are slightly larger, more rounded, much less mitotically active, and paler or less hyperchromatic. In rare cases, these rosettes may aggregate in a sheet of back-to-back clusters resembling field stone pavement.[2]
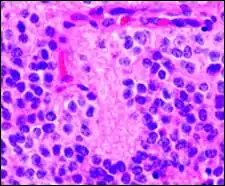 Micrograph of pineocytomatous/neurocytic pseudorosettes
Micrograph of pineocytomatous/neurocytic pseudorosettes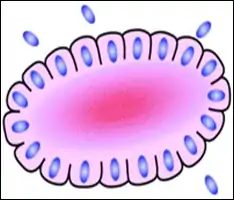 Structure of pineocytomatous/neurocytic pseudorosettes
Structure of pineocytomatous/neurocytic pseudorosettes
Clinical significance of rosettes and pseudorosettes
The neuropathologic diagnosis of brain tumors entails the microscopic examination of conventional formalin-fixed paraffin-embedded tissue samples surgically removed from a radiographically defined lesion. Pathologists rely on visual clues such as pattern recognition when examining the stained tissue with a microscope, much as radiologists rely on grayscale patterns of densities and intensities on images. Some histologic patterns of cellular architecture are distinctive if not pathognomonic whereas others are less specific, but nevertheless considerably narrow the differential diagnosis. The precise biologic bases for some of the observed microscopic patterns are poorly understood though their recognition remains useful nonetheless. One commonly encountered neuropathologic histologic architectural pattern seen within certain tumors is the rosette. The purpose of this report is to review the patterns of rosettes and pseudorosettes in the context of such tumors as medulloblastoma/primitive neuroectodermal tumor (PNET), retinoblastoma, ependymoma, central neurocytoma and pineocytoma.[2]
Methods for diagnosis of rosettes and pseudorosettes
More advanced methods of tissue examination such as histochemical and immunohistochemical profiling, genetic analysis, and electron microscopy have been developed, the microscopic review of H&E stained material remains a critical component of tumor diagnosis. Immunohistochemical evidence of neuronal differentiation is found in nearly all cases with neuronal markers such as synaptophysin, neuronspecific enolase, and neurofilament protein. Some medulloblastomas may also display other forms of differentiation as demonstrated by the presence of the astrocytic marker glial fibrillary acidic protein. Skeletal muscle and melanocytic differentiation are considerably less common and define the medullomyoblastoma and melanotic medulloblastoma variants, respectively.[2]
Long palisades
Palisades that are generally longer than a rosette or pseudorosette can be seen in neural tumors such as Schwannoma,[16][17] as well as in ameloblastomas. It can also be seen in nodular basal-cell carcinomas.[18]
Visually similar findings
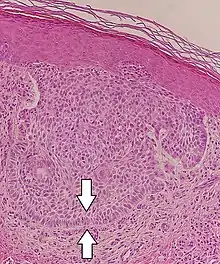
Pseudopalisading, a visually similar finding, is the formation of hypercellular zones that typically surrounds necrotic tissue.
 Pseudopalisades seen around necroses in glioblastoma.
Pseudopalisades seen around necroses in glioblastoma.
References
- "palisading". The Free Dictionary by Farlex, citing Segen's Medical Dictionary, copyrighted 2012. Retrieved 2019-09-11.
- Largely copied from: Ahmed, Mahtab Uddin (2017). "Rosettes and Pseudorosettes and Their Significance". Journal of Enam Medical College. 7 (2): 101–106. doi:10.3329/jemc.v7i2.32656. ISSN 2304-9316. Attribution 4.0 International (CC BY 4.0)
- Definition of 'rosette', from The Free Dictionary. Retrieved 6 January 2010.
- page 666 in: Ben Z. Pilch (2001). Head and Neck Surgical Pathology. Lippincott Williams & Wilkins. ISBN 9780397517275.
- McLean IW, Burnier MN, Zimmerman LE, et al. Tumors of the retina. In: Atlas of tumor pathology: tumors of the eye and ocular adnexa. Washington, DC: Armed Forces Institute of Pathology; 1994:97–154
- Donoso LA, Shields CL, Lee EY-H. Immunohistochemistry of retinoblastoma. Ophthal Paediatr Genet 1989;10:3–32
- Vrabec T, Arbizo V, Adamus G, et al. Rod cell-specific antigens in retinoblastoma. Arch Ophthalmol 1989;107:1061–63
- Kivela T. Glycoconjugates in retinoblastoma: a lectin histochemical study of ten formalin-fixed and paraffin embedded tumours. Virchows Arch A 1987;410:471–79
- Ts’o MOM, Fine BS, Zimmerman LE. The Flexner–Wintersteiner rosettes in retinoblastoma. Arch Pathol 1969;88:664–71
- Zimmerman LE. Retinoblastoma and retinocytoma. In: Spencer WH, ed. Ophthalmic pathology: an atlas and textbook. Philadelphia: WB Saunders; 1985:1292–351
- Flexner S. A peculiar glioma (neuroepithelioma?) of the retina. Johns Hopkins Hosp Bull 1891;2:115–19
- Schatski SC. Simon Flexner. AJR Am J Roentgenol 1997;169:1395–96.
- Bendiner E. Simon Flexner: his "rock" was for the ages. Hosp Pract 1988;23:213–66
- Wintersteiner H. Die zellen der geschwulst. In: Das neuroepithelioma retinae: Eine anatomische und klinishe studie. Leipzig: Franz Deuticke; 1897:12–16
- Wippold II, Franz J.; Perry, A. (March 2006). "Neuropathology for the Neuroradiologist: Rosettes and Pseudorosettes". American Journal of Neuroradiology. 27 (3): 488–492. PMID 16551982. Retrieved 26 August 2015.
- Wippold FJ, Lämmle M, Anatelli F, Lennerz J, Perry A (2006). "Neuropathology for the neuroradiologist: palisades and pseudopalisades". AJNR Am J Neuroradiol. 27 (10): 2037–41. PMID 17110662.
- Kadono T, Okada H, Okuno T, Ohara K (June 1998). "Basal cell carcinoma with neuroid type nuclear palisading: a report of three cases". Br. J. Dermatol. 138 (6): 1064–6. doi:10.1046/j.1365-2133.1998.02281.x. PMID 9747376. S2CID 20339424.
- Initially copied from: Paolino, Giovanni; Donati, Michele; Didona, Dario; Mercuri, Santo; Cantisani, Carmen (2017). "Histology of Non-Melanoma Skin Cancers: An Update". Biomedicines. 5 (4): 71. doi:10.3390/biomedicines5040071. ISSN 2227-9059. PMC 5744095. PMID 29261131.