Palovarotene
Palovarotene, sold under the brand name Sohonos, is a medication used for the treatment of heterotopic ossification (HO) and fibrodysplasia ossificans progressiva (FOP). It is a highly selective retinoic acid receptor gamma (RARγ) agonist.[3][4]
 | |
| Clinical data | |
|---|---|
| Trade names | Sohonos |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
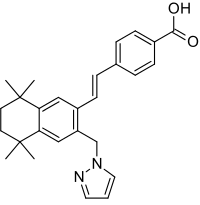
| Formula | C27H30N2O2 |
| Molar mass | 414.549 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Palovarotene is being developed by Ipsen Biopharmaceuticals and was granted Fast Track and orphan drug designations by the United States Food and Drug Administration for the treatment of FOP and Orphan Medicinal Product Designation by the European Medicines Agency (EMA) in 2014.[5][6][7] Phase II clinical studies yielded positive results.[8][9]
History
Palovarotene is a retinoic acid receptor gamma (RARγ) agonist licensed to Clementia Pharmaceuticals from Roche Pharmaceuticals. At Roche, palovarotene was evaluated in more than 800 individuals including healthy volunteers and patients with chronic obstructive pulmonary disease (COPD).[10] A one-year trial did not demonstrate a significant benefit on lung density in moderate-to-severe emphysema secondary to severe α(1)-antitrypsin deficiency.[11]
In 2011, animal studies demonstrated that RARγ agonists, including palovarotene, blocked new bone formation in both an injury-induced mouse model of heterotopic ossification (HO) and a genetically modified biological mouse model of FOP containing a continuously active ACVR1/ALK2 receptor in a dose-dependent manner.[12][13] A 2016 study demonstrated that palovarotene also inhibited spontaneous heterotopic ossification, maintained limb mobility and functioning, and restored skeletal growth in FOP mouse models.[14]
Research
Phase II
Clementia submitted a new drug application for palovarotene for the treatment of FOP after observing positive phase II results.[15]
References
- "Notice: Multiple Additions to the Prescription Drug List (PDL) [2022-01-24]". Health Canada. 24 January 2022. Retrieved 28 May 2022.
- "Summary Basis of Decision - Sohonos". Health Canada. 23 October 2014. Retrieved 6 August 2022.
- "FOP Fact Sheets". www.ifopa.org. Archived from the original on 4 April 2016. Retrieved 11 April 2016.
- "Health Canada Approves Ipsen's Sohonos (palovarotene capsules) as the First Approved Treatment for Fibrodysplasia Ossificans Progressiva" (Press release). Ipsen. 24 January 2022. Retrieved 28 May 2022 – via Business Wire.
- "Public summary of opinion on orphan designation. Palovarotene for the treatment of fibrodysplasia ossificans progressiva" (PDF). www.ema.europa.eu. Committee for Orphan Medicinal Products. Retrieved 11 April 2016.
- "Clementia Pharmaceuticals Receives Fast Track Designation for Palovarotene for Treatment of Fibrodysplasia Ossificans Progressiva (FOP)". PRNewswise. 1 December 2014. Retrieved 11 April 2016.
- "Clementia Pharmaceuticals Receives EMA Orphan Medicinal Product Designation for Palovarotene for the Treatment of Fibrodysplasia Ossificans Progressiva". PRNewswire. 21 November 2014. Retrieved 11 April 2016.
- "Clementia Pharmaceuticals Initiates Phase 2 Study of Palovarotene in Patients With Fibrodysplasia Ossificans Progressiva (FOP)". Marketwired. 14 July 2014. Archived from the original on 22 April 2016. Retrieved 11 April 2016.
- Marriott N (October 16, 2016). "Success for Clementia's Phase II Fibrodysplasia Ossificans Progressiva trial". European Pharmaceutical Review.
- Hind M, Stinchcombe S (November 2009). "Palovarotene, a novel retinoic acid receptor gamma agonist for the treatment of emphysema". Current Opinion in Investigational Drugs. 10 (11): 1243–50. PMID 19876792.
- Stolk J, Stockley RA, Stoel BC, Cooper BG, Piitulainen E, Seersholm N, et al. (August 2012). "Randomised controlled trial for emphysema with a selective agonist of the γ-type retinoic acid receptor". The European Respiratory Journal. 40 (2): 306–12. doi:10.1183/09031936.00161911. PMID 22282548.
- Shimono K, Tung WE, Macolino C, Chi AH, Didizian JH, Mundy C, et al. (April 2011). "Potent inhibition of heterotopic ossification by nuclear retinoic acid receptor-γ agonists". Nature Medicine. 17 (4): 454–60. doi:10.1038/nm.2334. PMC 3073031. PMID 21460849.
- Kaplan FS, Shore EM (April 2011). "Derailing heterotopic ossification and RARing to go". Nature Medicine. 17 (4): 420–1. doi:10.1038/nm0411-420. PMC 4913781. PMID 21475232.
- Chakkalakal SA, Uchibe K, Convente MR, Zhang D, Economides AN, Kaplan FS, et al. (September 2016). "Palovarotene Inhibits Heterotopic Ossification and Maintains Limb Mobility and Growth in Mice With the Human ACVR1(R206H) Fibrodysplasia Ossificans Progressiva (FOP) Mutation". Journal of Bone and Mineral Research. 31 (9): 1666–75. doi:10.1002/jbmr.2820. PMC 4992469. PMID 26896819.
- "Clementia Announces Plan to Submit a New Drug Application for Palovarotene for the Treatment of FOP Based on Positive Phase 2 Results". 23 October 2018.
- "Ipsen Initiates Partial Clinical Hold for Palovarotene IND120181 and IND135403 Studies".
- "Ipsen Completes Acquisition of Clementia Pharmaceuticals".