Perifosine
Perifosine (also KRX-0401) is a former drug candidate that was under development for a variety of cancer indications. It is an alkyl-phospholipid[1] structurally related to miltefosine. Perifosine interrupts the PI3K/AKT/mTOR pathway by acting as an allosteric AKT inhibitor targeting the pleckstrin homology domain of AKT.[2] It was being developed by Keryx Biopharmaceuticals who had licensed it from Æterna Zentaris Inc.[3]
 | |
 | |
| Names | |
|---|---|
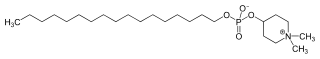
| IUPAC name
1,1-Dimethylpiperidinium-4-yl octadecyl phosphate | |
| Other names
D 21266; KRX 0401 | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.217.789 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C25H52NO4P |
| Molar mass | 461.668 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
In 2010, perifosine received orphan drug status in the U.S. for the treatment of multiple myeloma and neuroblastoma, and for multiple myeloma in the EU.[4] However, both were later withdrawn.[5][6]
In 2011 it was in a phase III trial for colorectal cancer,[7] and another for multiple myeloma.[4][8] On April 2, 2012, it was announced that perifosine failed its phase III clinical trial for treatment of colon cancer.[9] Detailed results were released in June 2012.[10] On March 11, 2013 Aeterna Zentaris announced the discontinuing of Phase 3 clinical trial of perifosine for the treatment of relapsed and refractory multiple myeloma.[11]
References
- Kondapaka; et al. (Nov 2003). "Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation". Molecular Cancer Therapeutics. Mol Cancer Ther. 2 (11): 1093–103. PMID 14617782.
- Keane, N. A.; Glavey, S. V.; Krawczyk, J.; O'Dwyer, M. (2014). "AKT as a therapeutic target in multiple myeloma". Expert Opinion on Therapeutic Targets. 18 (8): 897–915. doi:10.1517/14728222.2014.924507. PMID 24905897. S2CID 873910.
- Smartoncology newsletter Archived 2011-07-16 at the Wayback Machine, Feb 2010
- "Yakult Pays Aeterna Zentaris $8.3M for Japanese Rights to Pivotal-Stage Cancer Drug". 9 March 2011.
- "FDA Orphan Drug Designations and Approvals: Perifosine".
- "EU/3/10/740: Orphan designation for the treatment of multiple myeloma: Perifosine".
- "Archived copy". Archived from the original on 2011-07-27. Retrieved 2011-03-24.
{{cite web}}: CS1 maint: archived copy as title (link) - Clinical trial number NCT01002248 for "Assessment of Efficacy and Safety of Perifosine, Bortezomib and Dexamethasone in Multiple Myeloma Patients" at ClinicalTrials.gov
- "Aeterna Zentaris Regains North American Rights to Akt Inhibitor from Keryx". May 2012.
- "Aeterna Zentaris: Phase 3 Data for Perifosine in Colorectal Cancer Presented at ASCO Meeting". 4 June 2012. Archived from the original on 16 January 2013. Retrieved 13 November 2012.
- "Æterna Zentaris Inc". Archived from the original on 2014-02-02. Retrieved 2014-01-22.