Peripheral T-cell lymphoma not otherwise specified
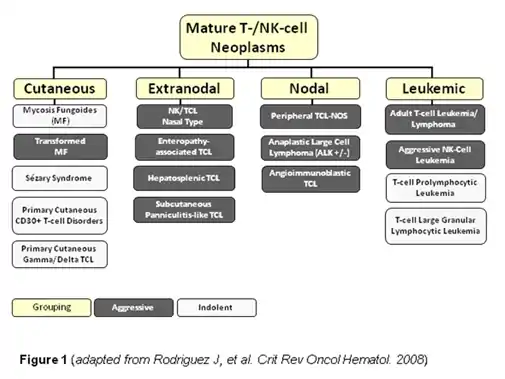
Peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), is a subtype of peripheral T-cell lymphoma. Peripheral T-cell lymphoma (PTCL) is defined as a diverse group of aggressive lymphomas that develop from mature-stage white blood cells called T-cells and natural killer cells (NK cells) (see figure for an overview of PTCL subtypes). PTCL is a type of non-Hodgkin's lymphoma (NHL).[2] PTCL specifically affects T-cells rather than B-cells, and results when T-cells develop and grow abnormally.
| Peripheral T-cell lymphoma-Not-Otherwise-Specified | |
|---|---|
| Specialty | Oncology |
About 30% of PTCL-NOS cases exhibit malignant T cells that are infected with the Epstein-Barr virus (EBV). When associated with EBV, PTCL-NOS is classified as one of the Epstein-Barr virus-associated lymphoproliferative diseases (see Epstein-Barr virus-associated peripheral T cell lymphoma, not otherwise specified) but the relationship of EBV to the development and progression of Epstein-Barr virus-associated PTCL-NOS is unclear.[3]
PTCL-NOS, the most common subtype of PTCL, is aggressive and predominantly nodal. There are two morphologic variants: the T-zone lymphoma variant and the lymphoepithelioid cell variant.[4][5]
- T-zone lymphoma is so named for its involvement in a specific area of the lymph node that consists of a dense accumulation of T-cells.[6]
- Lympho-epithelioid lymphoma, also called Lennert's lymphoma, is rare and generally affects older individuals.[7]
Treatment
Currently PTCL is treated similarly to B-cell lymphomas. However, in recent years, scientists have developed techniques to better recognize the different types of lymphomas, such as PTCL. It is now understood that PTCL behaves differently from B-cell lymphomas and therapies are being developed that specifically target these types of lymphoma. Currently, however, there are no therapies approved by the US Food and Drug Administration (FDA) specifically for PTCL. Anthracycline-containing chemotherapy regimens are commonly offered as the initial therapy. Some patients may receive a stem cell transplant.[8][9][10][11][12][13][14][15] Novel approaches to the treatment of PTCL in the relapsed or refractory setting are under investigation.
Pralatrexate and cerdulatinib are some of the compounds currently under investigations for the treatment of PTC.
References
- Rodriguez, J.; Gutierrez, A.; Martinez-Delgado, B.; Perez-Manga, G. (2009). "Current and future aggressive peripheral T-cell lymphoma treatment paradigms, biological features and therapeutic molecular targets". Crit Rev Oncol Hematol. 71 (3): 181–198. doi:10.1016/j.critrevonc.2008.10.011. PMID 19056295.
- Swerdlow SH, WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 2008
- Rezk SA, Zhao X, Weiss LM (June 2018). "Epstein - Barr virus - associated lymphoid proliferations, a 2018 update". Human Pathology. 79: 18–41. doi:10.1016/j.humpath.2018.05.020. PMID 29885408.
- Vose JM (October 2008). "Peripheral T-cell non-Hodgkin's lymphoma". Hematol. Oncol. Clin. North Am. 22 (5): 997–1005, x. doi:10.1016/j.hoc.2008.07.010. PMID 18954748.
- O’Connor, Owen. Getting the Facts; Peripheral T-Cell Lymphoma. [electronic version] Retrieved May 19, 2009, from http://www.lymphoma.org/atf/cf/%7B0363CDD6-51B5-427B-BE48-E6AF871ACEC9%7D/PTCL.PDF
- Stein H, Bonk A, Tolksdorf G, Lennert K, Rodt H, Gerdes J (August 1980). "Immunohistologic analysis of the organization of normal lymphoid tissue and non-Hodgkin's lymphomas". J. Histochem. Cytochem. 28 (8): 746–60. doi:10.1177/28.8.7003001. PMID 7003001.
- Daneshbod Y (2006). "Cytologic findings of peripheral T-cell lymphoma (PTCL) with high epitheloid cell content (Lennert's lymphoma) in imprint smear. A case report". CytoJournal. 3: 3. doi:10.1186/1742-6413-3-3. PMC 1434763. PMID 16460569.
- Reimer P, Rüdiger T, Geissinger E, et al. (January 2009). "Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study". J. Clin. Oncol. 27 (1): 106–13. doi:10.1200/JCO.2008.17.4870. PMID 19029417. Archived from the original on August 3, 2012. Retrieved July 6, 2011.
- Mercadal S, Briones J, Xicoy B, et al. (May 2008). "Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma". Ann. Oncol. 19 (5): 958–63. doi:10.1093/annonc/mdn022. PMID 18303032.
- Rodríguez J, Conde E, Gutiérrez A, et al. (July 2007). "Frontline autologous stem cell transplantation in high-risk peripheral T-cell lymphoma: a prospective study from The Gel-Tamo Study Group". Eur. J. Haematol. 79 (1): 32–8. doi:10.1111/j.1600-0609.2007.00856.x. PMID 17598836.
- Corradini P, Tarella C, Zallio F, et al. (September 2006). "Long-term follow-up of patients with peripheral T-cell lymphomas treated up-front with high-dose chemotherapy followed by autologous stem cell transplantation". Leukemia. 20 (9): 1533–8. doi:10.1038/sj.leu.2404306. PMID 16871285.
- d’Amore F, et al. Blood. 2006;108:A401
- Gisselbrecht C, Lepage E, Molina T, et al. (May 2002). "Shortened first-line high-dose chemotherapy for patients with poor-prognosis aggressive lymphoma". J. Clin. Oncol. 20 (10): 2472–9. doi:10.1200/JCO.2002.02.125. PMID 12011124. Archived from the original on September 3, 2012.
- Deconinck E, Lamy T, Foussard C, et al. (June 2000). "Autologous stem cell transplantation for anaplastic large-cell lymphomas: results of a prospective trial". Br. J. Haematol. 109 (4): 736–42. doi:10.1046/j.1365-2141.2000.02098.x. PMID 10929023.
- Haioun C, Lepage E, Gisselbrecht C, et al. (August 2000). "Survival benefit of high-dose therapy in poor-risk aggressive non-Hodgkin's lymphoma: final analysis of the prospective LNH87-2 protocol—a groupe d'Etude des lymphomes de l'Adulte study". J. Clin. Oncol. 18 (16): 3025–30. doi:10.1200/JCO.2000.18.16.3025. PMID 10944137. Archived from the original on September 6, 2012.