Extremophile
An extremophile (from Latin extremus meaning "extreme" and Greek philiā (φιλία) meaning "love") is an organism that is able to live (or in some cases thrive) in extreme environments, i.e. environments that make survival challenging such as due to extreme temperature, radiation, salinity, or pH level.[1]

These organisms are ecologically dominant in the evolutionary history of the planet. Some spores and cocooned bacteria samples have been dormant for more than 40 million years , extremophiles have continued to thrive in the most extreme conditions, making them one of the most abundant lifeforms.[2]
Characteristics
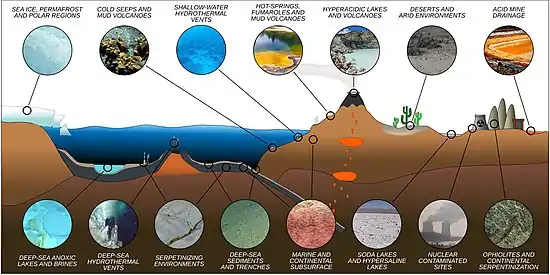
In the 1980s and 1990s, biologists found that microbial life has great flexibility for surviving in extreme environments—niches that are acidic, extraordinarily hot or within irregular air pressure for example—that would be completely inhospitable to complex organisms. Some scientists even concluded that life may have begun on Earth in hydrothermal vents far under the ocean's surface.[4]
According to astrophysicist Steinn Sigurdsson, "There are viable bacterial spores that have been found that are 40 million years old on Earth—and we know they're very hardened to radiation."[5] Some bacteria were found living in the cold and dark in a lake buried a half-mile deep under the ice in Antarctica,[6] and in the Marianas Trench, the deepest place in Earth's oceans.[7][8] Expeditions of the International Ocean Discovery Program found microorganisms in 120 °C sediment that is 1.2 km below seafloor in the Nankai Trough subduction zone.[9][10] Some microorganisms have been found thriving inside rocks up to 1,900 feet (580 m) below the sea floor under 8,500 feet (2,600 m) of ocean off the coast of the northwestern United States.[7][11] According to one of the researchers, "You can find microbes everywhere—they're extremely adaptable to conditions, and survive wherever they are."[7] A key to extremophile adaptation is their amino acid composition, affecting their protein folding ability under particular conditions.[12] Studying extreme environments on Earth can help researchers understand the limits of habitability on other worlds.[13]
Tom Gheysens from Ghent University in Belgium and some of his colleagues have presented research findings that show spores from a species of Bacillus bacteria survived and were still viable after being heated to temperatures of 420 °C (788 °F).[14]
| Limits of known life on Earth[15] | |||
|---|---|---|---|
| Factor | Environment / source | Limits | Examples |
| High temperature | Submarine hydrothermal vents, oceanic crust | 110 °C (230 °F) to 121 °C (250 °F)[9][15] | Pyrolobus fumarii, Pyrococcus furiosus |
| Low temperature | Ice | −20 °C (−4 °F) to −25 °C (−13 °F)[16] | Rhodotorula glutinis |
| Alkaline systems | Soda lakes | pH > 11[15] | Psychrobacter, Vibrio, Arthrobacter, Natronobacterium |
| Acidic systems | Volcanic springs, acid mine drainage | pH -0.06 to 1.0[15] | Picrophilus |
| Ionizing radiation | Cosmic rays, X-rays, radioactive decay | 1,500 to 6,000 Gy[15] | Deinococcus radiodurans, Rubrobacter, Thermococcus gammatolerans |
| UV radiation | Sunlight | 5,000 J/m2[15] | |
| High pressure | Mariana Trench | 1,100 bar[15] | Pyrococcus sp. |
| Salinity | High salt concentration | aw ~ 0.6[15] | Halobacteriaceae, Dunaliella salina |
| Desiccation | Atacama Desert (Chile), McMurdo Dry Valleys (Antarctica) | ~60% relative humidity[15] | Chroococcidiopsis |
| Deep crust | Accessed in some gold mines | Halicephalobus mephisto, Mylonchulus brachyurus, unidentified arthropods | |
Classifications
There are many classes of extremophiles that range all around the globe; each corresponding to the way its environmental niche differs from mesophilic conditions. These classifications are not exclusive. Many extremophiles fall under multiple categories and are classified as polyextremophiles. For example, organisms living inside hot rocks deep under Earth's surface are thermophilic and piezophilic such as Thermococcus barophilus.[17] A polyextremophile living at the summit of a mountain in the Atacama Desert might be a radioresistant xerophile, a psychrophile, and an oligotroph. Polyextremophiles are well known for their ability to tolerate both high and low pH levels.[18]
Terms
- Acidophile
- An organism with optimal growth at pH levels of 3.0 or below.
- Alkaliphile
- An organism with optimal growth at pH levels of 9.0 or above.
- Anaerobe
- An organism with optimal growth in the absence of molecular oxygen. Two sub-types exist: facultative anaerobe and obligate anaerobe. A facultative anaerobe can tolerate anoxic and oxic conditions whilst an obligate anaerobe will die in the presence of even low levels of molecular oxygen.:
- Capnophile
- An organism with optimal growth conditions in high concentrations of carbon dioxide. An example would be Mannheimia succiniciproducens, a bacterium that inhabits a ruminant animal's digestive system.[19]
- Cryptoendolith
- An organism that lives in microscopic spaces within rocks, such as pores between aggregate grains. These may also be called endolith, a term that also includes organisms populating fissures, aquifers, and faults filled with groundwater in the deep subsurface.
- Halophile
- An organism with optimal growth at a concentration of dissolved salts of 50 g/L (= 5% m/v) or above.
- Hyperpiezophile
- An organism with optimal growth at hydrostatic pressures above 50 MPa (= 493 atm = 7,252 psi).
- Hyperthermophile
- An organism with optimal growth at temperatures above 80 °C (176 °F).
- Hypolith
- An organism that lives underneath rocks in cold deserts.
- Metallotolerant
- Capable of tolerating high levels of dissolved heavy metals in solution, such as copper, cadmium, arsenic, and zinc. Examples include Ferroplasma sp., Cupriavidus metallidurans and GFAJ-1.[20][21][22]
- Oligotroph
- An organism with optimal growth in nutritionally limited environments.
- Osmophile
- An organism with optimal growth in environments with a high sugar concentration.
- Piezophile
- An organism with optimal growth in hydrostatic pressures above 10 MPa (= 99 atm = 1,450 psi). Also referred to as barophile.
- Polyextremophile
- A polyextremophile (faux Ancient Latin/Greek for 'affection for many extremes') is an organism that qualifies as an extremophile under more than one category.
- Psychrophile/Cryophile
- An organism with optimal growth at temperatures of 15 °C (59 °F) or lower.
- Radioresistant
- Organisms resistant to high levels of ionizing radiation, most commonly ultraviolet radiation. This category also includes organisms capable of resisting nuclear radiation.
- Sulphophile
- An organism with optimal growth conditions in high concentrations of sulfur. An example would be Sulfurovum Epsilonproteobacteria, a sulfur-oxidizing bacteria that inhabits deep-water sulfur vents.[23]
- Thermophile
- An organism with optimal growth at temperatures above 45 °C (113 °F).
- Xerophile
- An organism with optimal growth at water activity below 0.8.
In astrobiology
Astrobiology is the multidisciplinary field that investigates the deterministic conditions and contingent events with which life arises, distributes, and evolves in the universe. Astrobiology makes use of physics, chemistry, astronomy, solar physics, biology, molecular biology, ecology, planetary science, geography, and geology to investigate the possibility of life on other worlds and help recognize biospheres that might be different from that on Earth.[24] Astrobiologists are particularly interested in studying extremophiles, as it allows them to map what is known about the limits of life on Earth to potential extraterrestrial environments[1] For example, analogous deserts of Antarctica are exposed to harmful UV radiation, low temperature, high salt concentration and low mineral concentration. These conditions are similar to those on Mars. Therefore, finding viable microbes in the subsurface of Antarctica suggests that there may be microbes surviving in endolithic communities and living under the Martian surface. Research indicates it is unlikely that Martian microbes exist on the surface or at shallow depths, but may be found at subsurface depths of around 100 meters.[25]
Recent research carried out on extremophiles in Japan involved a variety of bacteria including Escherichia coli and Paracoccus denitrificans being subject to conditions of extreme gravity. The bacteria were cultivated while being rotated in an ultracentrifuge at high speeds corresponding to 403,627 g (i.e. 403,627 times the gravity experienced on Earth). Paracoccus denitrificans was one of the bacteria which displayed not only survival but also robust cellular growth under these conditions of hyperacceleration which are usually found only in cosmic environments, such as on very massive stars or in the shock waves of supernovas. Analysis showed that the small size of prokaryotic cells is essential for successful growth under hypergravity. The research has implications on the feasibility of panspermia.[26][27][28]
On 26 April 2012, scientists reported that lichen survived and showed remarkable results on the adaptation capacity of photosynthetic activity within the simulation time of 34 days under Martian conditions in the Mars Simulation Laboratory (MSL) maintained by the German Aerospace Center (DLR).[29][30]
On 29 April 2013, scientists at Rensselaer Polytechnic Institute, funded by NASA, reported that, during spaceflight on the International Space Station, microbes seem to adapt to the space environment in ways "not observed on Earth" and in ways that "can lead to increases in growth and virulence".[31]
On 19 May 2014, scientists announced that numerous microbes, like Tersicoccus phoenicis, may be resistant to methods usually used in spacecraft assembly clean rooms. It's not currently known if such resistant microbes could have withstood space travel and are present on the Curiosity rover now on the planet Mars.[32]
On 20 August 2014, scientists confirmed the existence of microorganisms living half a mile below the ice of Antarctica.[33][34]
In September 2015, scientists from CNR-National Research Council of Italy reported that S.soflataricus was able to survive under Martian radiation at a wavelength that was considered extremely lethal to most bacteria. This discovery is significant because it indicates that not only bacterial spores, but also growing cells can be remarkably resistant to strong UV radiation.[35]
In June 2016, scientists from Brigham Young University conclusively reported that endospores of Bacillus subtilis were able to survive high speed impacts up to 299±28 m/s, extreme shock, and extreme deceleration. They pointed out that this feature might allow endospores to survive and to be transferred between planets by traveling within meteorites or by experiencing atmosphere disruption. Moreover, they suggested that the landing of spacecraft may also result in interplanetary spore transfer, given that spores can survive high-velocity impact while ejected from the spacecraft onto the planet surface. This is the first study which reported that bacteria can survive in such high-velocity impact. However, the lethal impact speed is unknown, and further experiments should be done by introducing higher-velocity impact to bacterial endospores.[36]
In August 2020 scientists reported that bacteria that feed on air discovered 2017 in Antarctica are likely not limited to Antarctica after discovering the two genes previously linked to their "atmospheric chemosynthesis" in soil of two other similar cold desert sites, which provides further information on this carbon sink and further strengthens the extremophile evidence that supports the potential existence of microbial life on alien planets.[37][38][39]
The same month, scientists reported that bacteria from Earth, particularly Deinococcus radiodurans, were found to survive for three years in outer space, based on studies on the International Space Station. These findings support the notion of panspermia.[40][41]
Bioremediation
Extremophiles can also be useful players in the bioremediation of contaminated sites as some species are capable of biodegradation under conditions too extreme for classic bioremediation candidate species. Anthropogenic activity causes the release of pollutants that may potentially settle in extreme environments as is the case with tailings and sediment released from deep-sea mining activity.[42] While most bacteria would be crushed by the pressure in these environments, piezophiles can tolerate these depths and can metabolize pollutants of concern if they possess bioremediation potential.
Hydrocarbons
There are multiple potential destinations for hydrocarbons after an oil spill has settled and currents routinely deposit them in extreme environments. Methane bubbles resulting from the Deepwater Horizon oil spill were found 1.1 kilometers below water surface level and at concentrations as high as 183 μmol per kilogram.[43] The combination of low temperatures and high pressures in this environment result in low microbial activity. However, bacteria that are present including species of Pseudomonas, Aeromonas and Vibrio were found to be capable of bioremediation, albeit at a tenth of the speed they would perform at sea level pressure.[44] Polycyclic Aromatic Hydrocarbons increase in solubility and bioavailability with increasing temperature. Thermophilic Thermus and Bacillus species have demonstrated higher gene expression for the alkane mono-oxygenase alkB at temperatures exceeding 60 °C. The expression of this gene is a crucial precursor to the bioremediation process. Fungi that have been genetically modified with cold-adapted enzymes to tolerate differing pH levels and temperatures have been shown to be effective at remediating hydrocarbon contamination in freezing conditions in the Antarctic.[45]
Metals
Acidithiubacillus ferroxidans has been shown to be effective in remediating mercury in acidic soil due to its merA gene making it mercury resistant.[46] Industrial effluent contain high levels of metals that can be detrimental to both human and ecosystem health.[47][48] In extreme heat environments the extremophile Geobacillus thermodenitrificans has been shown to effectively manage the concentration of these metals within twelve hours of introduction.[49] Some acidophilic microorganisms are effective at metal remediation in acidic environments due to proteins found in their periplasm, not present in any mesophilic organisms, allowing them to protect themselves from high proton concentrations.[50] Rice paddies are highly oxidative environments that can produce high levels of lead or cadmium. Deinococcus radiodurans are resistant to the harsh conditions of the environment and are therefore candidate species for limiting the extent of contamination of these metals.[51]
Some bacteria are known to also use rare earth elements on their biological processes for example Methylacidiphilum fumariolicum, Methylorubrum extorquens and Methylobacterium radiotolerans are known to be able to use lanthanides as cofactors to increase their methanol dehydrogenase activity.
Acid mine drainage
Acid mine drainage is a major environmental concern associated with many metal mines. One of the most productive methods of its remediation is through the introduction of the extremophile organism Thiobacillus ferrooxidans.[52]
Radioactive materials
Any bacteria capable of inhabiting radioactive mediums can be classified as an extremophile. Radioresistant organisms are therefore critical in the bioremediation of radionuclides. Uranium is particularly challenging to contain when released into an environment and very harmful to both human and ecosystem health.[53][54] The NANOBINDERS project is equipping bacteria that can survive in uranium rich environments with gene sequences that enable proteins to bind to uranium in mining effluent, making it more convenient to collect and dispose of.[55] Some examples are Shewanella putrefaciens, Geobacter metallireducens and some strains of Burkholderia fungorum.
Radiotrophic fungus, which use radiation as an energy source have been found inside and around the Chernobyl Nuclear Power Plant.[56]
Radioresistance has also been observed in certain species of macroscopic lifeforms. The lethal dose required to kill up to 50% of a tortoise population is 40,000 roentgens, compared to only 800 roentgens needed to kill 50% of a human population.[57] In experiments exposing lepidopteran insects to gamma radiation, significant DNA damage was detected only at 20 Gy and higher doses, in contrast with human cells that showed similar damage at only 2 Gy.[58]
Examples and recent findings
New sub-types of -philes are identified frequently and the sub-category list for extremophiles is always growing. For example, microbial life lives in the liquid asphalt lake, Pitch Lake. Research indicates that extremophiles inhabit the asphalt lake in populations ranging between 106 to 107 cells/gram.[59][60] Likewise, until recently boron tolerance was unknown but a strong borophile was discovered in bacteria. With the recent isolation of Bacillus boroniphilus, borophiles came into discussion.[61] Studying these borophiles may help illuminate the mechanisms of both boron toxicity and boron deficiency.
In July 2019, a scientific study of Kidd Mine in Canada discovered sulfur-breathing organisms which live 7900 feet below the surface, and which breathe sulfur in order to survive. These organisms are also remarkable due to eating rocks such as pyrite as their regular food source.[62][63][64]
Biotechnology
The thermoalkaliphilic catalase, which initiates the breakdown of hydrogen peroxide into oxygen and water, was isolated from an organism, Thermus brockianus, found in Yellowstone National Park by Idaho National Laboratory researchers. The catalase operates over a temperature range from 30 °C to over 94 °C and a pH range from 6–10. This catalase is extremely stable compared to other catalases at high temperatures and pH. In a comparative study, the T. brockianus catalase exhibited a half life of 15 days at 80 °C and pH 10 while a catalase derived from Aspergillus niger had a half life of 15 seconds under the same conditions. The catalase will have applications for removal of hydrogen peroxide in industrial processes such as pulp and paper bleaching, textile bleaching, food pasteurization, and surface decontamination of food packaging.[65]
DNA modifying enzymes such as Taq DNA polymerase and some Bacillus enzymes used in clinical diagnostics and starch liquefaction are produced commercially by several biotechnology companies.[66]
DNA transfer
Over 65 prokaryotic species are known to be naturally competent for genetic transformation, the ability to transfer DNA from one cell to another cell followed by integration of the donor DNA into the recipient cell's chromosome.[67] Several extremophiles are able to carry out species-specific DNA transfer, as described below. However, it is not yet clear how common such a capability is among extremophiles.
The bacterium Deinococcus radiodurans is one of the most radioresistant organisms known. This bacterium can also survive cold, dehydration, vacuum and acid and is thus known as a polyextremophile. D. radiodurans is competent to perform genetic transformation.[68] Recipient cells are able to repair DNA damage in donor transforming DNA that had been UV irradiated as efficiently as they repair cellular DNA when the cells themselves are irradiated. The extreme thermophilic bacterium Thermus thermophilus and other related Thermus species are also capable of genetic transformation.[69]
Halobacterium volcanii, an extreme halophilic (saline tolerant) archaeon, is capable of natural genetic transformation. Cytoplasmic bridges are formed between cells that appear to be used for DNA transfer from one cell to another in either direction.[70]
Sulfolobus solfataricus and Sulfolobus acidocaldarius are hyperthermophilic archaea. Exposure of these organisms to the DNA damaging agents UV irradiation, bleomycin or mitomycin C induces species-specific cellular aggregation.[71][72] UV-induced cellular aggregation of S. acidocaldarius mediates chromosomal marker exchange with high frequency.[72] Recombination rates exceed those of uninduced cultures by up to three orders of magnitude. Frols et al.[71] and Ajon et al.[72] hypothesized that cellular aggregation enhances species-specific DNA transfer between Sulfolobus cells in order to repair damaged DNA by means of homologous recombination. Van Wolferen et al.[73] noted that this DNA exchange process may be crucial under DNA damaging conditions such as high temperatures. It has also been suggested that DNA transfer in Sulfolobus may be an early form of sexual interaction similar to the more well-studied bacterial transformation systems that involve species-specific DNA transfer leading to homologous recombinational repair of DNA damage (and see Transformation (genetics)).
Extracellular membrane vesicles (MVs) might be involved in DNA transfer between different hyperthermophilic archaeal species.[74] It has been shown that both plasmids[75] and viral genomes[74] can be transferred via MVs. Notably, a horizontal plasmid transfer has been documented between hyperthermophilic Thermococcus and Methanocaldococcus species, respectively belonging to the orders Thermococcales and Methanococcales.[76]
See also
- Dissimilatory metal-reducing microorganisms
- Extremotroph
- List of microorganisms tested in outer space
- Mesophile – Organism that grows best in moderate temperatures
- Neutrophile – Organism that grows best in a neutral pH level
- RISE project
- Tardigrade
References
- Rothschild, Lynn; Mancinelli, Rocco (February 2001). "Life in extreme environments". Nature. 409 (6823): 1092–1101. Bibcode:2001Natur.409.1092R. doi:10.1038/35059215. PMID 11234023. S2CID 529873.
- Merino, Nancy; Aronson, Heidi S.; Bojanova, Diana P.; Feyhl-Buska, Jayme; Wong, Michael L.; Zhang, Shu; Giovannelli, Donato (2019). "Living at the Extremes: Extremophiles and the Limits of Life in a Planetary Context". Frontiers in Microbiology. 10: 780. doi:10.3389/fmicb.2019.00780. PMC 6476344. PMID 31037068. S2CID 115205576.
- Merino, Nancy; Aronson, Heidi S.; Bojanova, Diana P.; Feyhl-Buska, Jayme; Wong, Michael L.; Zhang, Shu; Giovannelli, Donato (2019). "Living at the Extremes: Extremophiles and the Limits of Life in a Planetary Context". Frontiers in Microbiology. 10: 780. doi:10.3389/fmicb.2019.00780. PMC 6476344. PMID 31037068.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - "Mars Exploration Rover Launches – Press kit" (PDF). NASA. June 2003. Retrieved 14 July 2009.
- BBC Staff (23 August 2011). "Impacts 'more likely' to have spread life from Earth". BBC. Retrieved 24 August 2011.
- Gorman J (6 February 2013). "Bacteria Found Deep Under Antarctic Ice, Scientists Say". The New York Times. Archived from the original on 1 January 2022. Retrieved 6 February 2013.
- Choi CQ (17 March 2013). "Microbes Thrive in Deepest Spot on Earth". LiveScience. Retrieved 17 March 2013.
- Glud RN, Wenzhöfer F, Middelboe M, Oguri K, Turnewitsch R, Canfield DE, Kitazato H (17 March 2013). "High rates of microbial carbon turnover in sediments in the deepest oceanic trench on Earth". Nature Geoscience. 6 (4): 284–88. Bibcode:2013NatGe...6..284G. doi:10.1038/ngeo1773.
- Heuer, Verena B.; Inagaki, Fumio; Morono, Yuki; Kubo, Yusuke; Spivack, Arthur J.; Viehweger, Bernhard; Treude, Tina; Beulig, Felix; Schubotz, Florence; Tonai, Satoshi; Bowden, Stephen A. (4 December 2020). "Temperature limits to deep subseafloor life in the Nankai Trough subduction zone". Science. 370 (6521): 1230–34. Bibcode:2020Sci...370.1230H. doi:10.1126/science.abd7934. hdl:2164/15700. ISSN 0036-8075. PMID 33273103. S2CID 227257205.
- "T-Limit of the Deep Biosphere off Muroto". www.jamstec.go.jp. Retrieved 8 March 2021.
- Oskin B (14 March 2013). "Intraterrestrials: Life Thrives in Ocean Floor". LiveScience. Retrieved 17 March 2013.
- Reed CJ, Lewis H, Trejo E, Winston V, Evilia C (2013). "Protein adaptations in archaeal extremophiles". Archaea. 2013: 373275. doi:10.1155/2013/373275. PMC 3787623. PMID 24151449.
- "NASA Astrobiology Strategy" (PDF). NASA. 2015. p. 59. Archived from the original (PDF) on 22 December 2016. Retrieved 12 October 2017.
- "Turn up the Heat: Bacterial Spores Can Take Temperatures in the Hundreds of Degrees".
- Marion, Giles M.; Fritsen, Christian H.; Eicken, Hajo; Payne, Meredith C. (December 2003). "The Search for Life on Europa: Limiting Environmental Factors, Potential Habitats, and Earth Analogues". Astrobiology. 3 (4): 785–811. Bibcode:2003AsBio...3..785M. doi:10.1089/153110703322736105. PMID 14987483.
- Neufeld, Josh; Clarke, Andrew; Morris, G. John; Fonseca, Fernanda; Murray, Benjamin J.; Acton, Elizabeth; Price, Hannah C. (2013). "A Low Temperature Limit for Life on Earth". PLOS ONE. 8 (6): e66207. Bibcode:2013PLoSO...866207C. doi:10.1371/journal.pone.0066207. PMC 3686811. PMID 23840425.
- Marteinsson VT, Birrien JL, Reysenbach AL, Vernet M, Marie D, Gambacorta A, Messner P, Sleytr UB, Prieur D (April 1999). "Thermococcus barophilus sp. nov., a new barophilic and hyperthermophilic archaeon isolated under high hydrostatic pressure from a deep-sea hydrothermal vent". International Journal of Systematic Bacteriology. 49 Pt 2 (2): 351–59. doi:10.1099/00207713-49-2-351. PMID 10319455.
- Yadav AN, Verma P, Kumar M, Pal KK, Dey R, Gupta A, et al. (31 May 2014). "Diversity and phylogenetic profiling of niche-specific Bacilli from extreme environments of India". Annals of Microbiology. 65 (2): 611–29. doi:10.1007/s13213-014-0897-9. S2CID 2369215.
- Hong, Soon Ho; Kim, Jin Sik; Lee, Sang Yup; In, Yong Ho; Choi, Sun Shim; Rih, Jeong-Keun; Kim, Chang Hoon; Jeong, Haeyoung; Hur, Cheol Goo; Kim, Jae Jong (19 September 2004). "The genome sequence of the capnophilic rumen bacterium Mannheimia succiniciproducens". Nature Biotechnology. 22 (10): 1275–81. doi:10.1038/nbt1010. ISSN 1087-0156. PMID 15378067. S2CID 35247112.
- "Studies refute arsenic bug claim". BBC News. 9 July 2012. Retrieved 10 July 2012.
- Erb TJ, Kiefer P, Hattendorf B, Günther D, Vorholt JA (July 2012). "GFAJ-1 is an arsenate-resistant, phosphate-dependent organism". Science. 337 (6093): 467–70. Bibcode:2012Sci...337..467E. doi:10.1126/science.1218455. PMID 22773139. S2CID 20229329.
- Reaves ML, Sinha S, Rabinowitz JD, Kruglyak L, Redfield RJ (July 2012). "Absence of detectable arsenate in DNA from arsenate-grown GFAJ-1 cells". Science. 337 (6093): 470–73. arXiv:1201.6643. Bibcode:2012Sci...337..470R. doi:10.1126/science.1219861. PMC 3845625. PMID 22773140.
- Meier, Dimitri V; Pjevac, Petra; Bach, Wolfgang; Hourdez, Stephane; Girguis, Peter R; Vidoudez, Charles; Amann, Rudolf; Meyerdierks, Anke (4 April 2017). "Niche partitioning of diverse sulfur-oxidizing bacteria at hydrothermal vents". The ISME Journal. 11 (7): 1545–58. doi:10.1038/ismej.2017.37. ISSN 1751-7362. PMC 5520155. PMID 28375213.
- Ward PD, Brownlee D (2004). The life and death of planet Earth. New York: Owl Books. ISBN 978-0805075120.
- Wynn-Williams DA, Newton EM, Edwards HG (2001). Exo-/astro-biology : proceedings of the first European workshop, 21–23 May 2001, ESRIN, Fracscati, Italy. Exo-/Astro-Biology. Vol. 496. p. 226. Bibcode:2001ESASP.496..225W. ISBN 978-92-9092-806-5.
- Than, Ker (25 April 2011). "Bacteria Grow Under 400,000 Times Earth's Gravity". National Geographic – Daily News. National Geographic Society. Retrieved 28 April 2011.
- Deguchi S, Shimoshige H, Tsudome M, Mukai SA, Corkery RW, Ito S, Horikoshi K (May 2011). "Microbial growth at hyperaccelerations up to 403,627 x g". Proceedings of the National Academy of Sciences of the United States of America. 108 (19): 7997–8002. Bibcode:2011PNAS..108.7997D. doi:10.1073/pnas.1018027108. PMC 3093466. PMID 21518884.
- Reuell, Peter (8 July 2019). "Harvard study suggests asteroids might play key role in spreading life". Harvard Gazette. Retrieved 6 October 2019.
- Baldwin E (26 April 2012). "Lichen survives harsh Mars environment". Skymania News. Retrieved 27 April 2012.
- De Vera JP, Kohler U (26 April 2012). "The adaptation potential of extremophiles to Martian surface conditions and its implication for the habitability of Mars" (PDF). EGU General Assembly Conference Abstracts. 14: 2113. Bibcode:2012EGUGA..14.2113D. Retrieved 27 April 2012.
- Kim W, Tengra FK, Young Z, Shong J, Marchand N, Chan HK, et al. (29 April 2013). "Spaceflight promotes biofilm formation by Pseudomonas aeruginosa". PLOS ONE. 8 (4): e62437. Bibcode:2013PLoSO...862437K. doi:10.1371/journal.pone.0062437. PMC 3639165. PMID 23658630.
- Madhusoodanan J (19 May 2014). "Microbial stowaways to Mars identified". Nature. doi:10.1038/nature.2014.15249. S2CID 87409424. Retrieved 23 May 2014.
- Fox D (August 2014). "Lakes under the ice: Antarctica's secret garden". Nature. 512 (7514): 244–46. Bibcode:2014Natur.512..244F. doi:10.1038/512244a. PMID 25143097.
- Mack E (20 August 2014). "Life Confirmed Under Antarctic Ice; Is Space Next?". Forbes. Retrieved 21 August 2014.
- Mastascusa V, Romano I, Di Donato P, Poli A, Della Corte V, Rotundi A, Bussoletti E, Quarto M, Pugliese M, Nicolaus B (September 2014). "Extremophiles survival to simulated space conditions: an astrobiology model study". Origins of Life and Evolution of the Biosphere. 44 (3): 231–37. Bibcode:2014OLEB...44..231M. doi:10.1007/s11084-014-9397-y. PMC 4669584. PMID 25573749.
- Barney BL, Pratt SN, Austin DE (June 2016). "Survivability of bare, individual Bacillus subtilis spores to high-velocity surface impact: Implications for microbial transfer through space". Planetary and Space Science. 125: 20–26. Bibcode:2016P&SS..125...20B. doi:10.1016/j.pss.2016.02.010.
- "Microbes living on air a global phenomenon". phys.org. Retrieved 8 September 2020.
- "Bacteria that "eat" only air found in cold deserts around the world". New Atlas. 19 August 2020. Retrieved 8 September 2020.
- Ray, Angelique E.; Zhang, Eden; Terauds, Aleks; Ji, Mukan; Kong, Weidong; Ferrari, Belinda C. (2020). "Soil Microbiomes With the Genetic Capacity for Atmospheric Chemosynthesis Are Widespread Across the Poles and Are Associated With Moisture, Carbon, and Nitrogen Limitation". Frontiers in Microbiology. 11: 1936. doi:10.3389/fmicb.2020.01936. ISSN 1664-302X. PMC 7437527. PMID 32903524. S2CID 221105556.
 Text and images are available under a Creative Commons Attribution 4.0 International License.
Text and images are available under a Creative Commons Attribution 4.0 International License. - Strickland, Ashley (26 August 2020). "Bacteria from Earth can survive in space and could endure the trip to Mars, according to new study". CNN News. Retrieved 26 August 2020.
- Kawaguchi, Yuko; et al. (26 August 2020). "DNA Damage and Survival Time Course of Deinococcal Cell Pellets During 3 Years of Exposure to Outer Space". Frontiers in Microbiology. 11: 2050. doi:10.3389/fmicb.2020.02050. PMC 7479814. PMID 32983036. S2CID 221300151.
 Text and images are available under a Creative Commons Attribution 4.0 International License.
Text and images are available under a Creative Commons Attribution 4.0 International License. - Frid, Christopher L. J.; Caswell, Bryony A. (23 November 2017). "Marine Pollution". Oxford Scholarship Online. doi:10.1093/oso/9780198726289.001.0001. ISBN 9780198726289.
- Reddy, C. M.; Arey, J. S.; Seewald, J. S.; Sylva, S. P.; Lemkau, K. L.; Nelson, R. K.; Carmichael, C. A.; McIntyre, C. P.; Fenwick, J.; Ventura, G. T.; Van Mooy, B. A. S. (18 July 2011). "Composition and fate of gas and oil released to the water column during the Deepwater Horizon oil spill". Proceedings of the National Academy of Sciences. 109 (50): 20229–34. doi:10.1073/pnas.1101242108. ISSN 0027-8424. PMC 3528605. PMID 21768331.
- Margesin, R.; Schinner, F. (1 September 2001). "Biodegradation and bioremediation of hydrocarbons in extreme environments". Applied Microbiology and Biotechnology. 56 (5–6): 650–63. doi:10.1007/s002530100701. ISSN 0175-7598. PMID 11601610. S2CID 13436065.
- Duarte, Alysson Wagner Fernandes; dos Santos, Juliana Aparecida; Vianna, Marina Vitti; Vieira, Juliana Maíra Freitas; Mallagutti, Vitor Hugo; Inforsato, Fabio José; Wentzel, Lia Costa Pinto; Lario, Luciana Daniela; Rodrigues, Andre; Pagnocca, Fernando Carlos; Pessoa Junior, Adalberto (11 December 2017). "Cold-adapted enzymes produced by fungi from terrestrial and marine Antarctic environments". Critical Reviews in Biotechnology. 38 (4): 600–19. doi:10.1080/07388551.2017.1379468. hdl:11449/175619. ISSN 0738-8551. PMID 29228814. S2CID 4201439.
- Takeuchi, Fumiaki; Iwahori, Kenji; Kamimura, Kazuo; Negishi, Atsunori; Maeda, Terunobu; Sugio, Tsuyoshi (January 2001). "Volatilization of Mercury under Acidic Conditions from Mercury-polluted Soil by a Mercury-resistant Acidithiobacillus ferrooxidans SUG 2-2". Bioscience, Biotechnology, and Biochemistry. 65 (9): 1981–86. doi:10.1271/bbb.65.1981. ISSN 0916-8451. PMID 11676009. S2CID 2158906.
- Nagajyoti, P.C. (2008). "Heavy metal toxicity: Industrial Effluent Effect on Groundnut (Arachis hypogaea L.) Seedlings". Journal of Applied Sciences Research. 4 (1): 110–21.
- Fakayode, S.O. (2005). "Impact assessment of industrial effluent on water quality of the receiving Alaro River in Ibadan, Nigeria". African Journal of Environmental Assessment and Management. 10: 1–13.
- Chatterjee, S.K.; Bhattacharjee, I.; Chandra, G. (March 2010). "Biosorption of heavy metals from industrial waste water by Geobacillus thermodenitrificans". Journal of Hazardous Materials. 175 (1–3): 117–25. doi:10.1016/j.jhazmat.2009.09.136. ISSN 0304-3894. PMID 19864059.
- Chi, A. (2007). "Periplasmic proteins of the extremophile Acidithiobacillus ferrooxidans: a high throughput proteomics analysis". Molecular & Cellular Proteomics. 6 (12): 2239–51. doi:10.1074/mcp.M700042-MCP200. PMC 4631397. PMID 17911085.
- Dai, Shang; Chen, Qi; Jiang, Meng; Wang, Binqiang; Xie, Zhenming; Yu, Ning; Zhou, Yulong; Li, Shan; Wang, Liangyan; Hua, Yuejin; Tian, Bing (September 2021). "Colonized extremophile Deinococcus radiodurans alleviates toxicity of cadmium and lead by suppressing heavy metal accumulation and improving antioxidant system in rice". Environmental Pollution. 284: 117127. doi:10.1016/j.envpol.2021.117127. ISSN 0269-7491. PMID 33892465.
- Akcil, Ata; Koldas, Soner (January 2006). "Acid Mine Drainage (AMD): causes, treatment and case studies". Journal of Cleaner Production. 14 (12–13): 1139–45. doi:10.1016/j.jclepro.2004.09.006. ISSN 0959-6526.
- "Toxicological Profile for Uranium", ATSDR's Toxicological Profiles, CRC Press, 12 January 2002, doi:10.1201/9781420061888_ch157, hdl:2027/mdp.39015032949136, ISBN 978-1-4200-6188-8
- Heising-Goodman, Carolyn (March 1981). "Nuclear Power and Its Environmental Effects". Nuclear Technology. 52 (3): 445. doi:10.13182/nt81-a32724. ISSN 0029-5450.
- Marques, Catarina R. (1 June 2018). "Extremophilic Microfactories: Applications in Metal and Radionuclide Bioremediation". Frontiers in Microbiology. 9: 1191. doi:10.3389/fmicb.2018.01191. ISSN 1664-302X. PMC 5992296. PMID 29910794.
- Castelvecchi, Davide (26 May 2007). "Dark Power: Pigment seems to put radiation to good use". Science News. Vol. 171, no. 21. p. 325. Archived from the original on 24 April 2008.
- "Tortoise blood fights radiation sickness".
- Chandna, S.; Dwarakanath, B. S.; Seth, R. K.; Khaitan, D.; Adhikari, J. S.; Jain, V. (2004). "Radiation responses of Sf9, a highly radioresistant lepidopteran insect cell line". International Journal of Radiation Biology. 80 (4): 301–315. doi:10.1080/09553000410001679794. PMID 15204707. S2CID 24978637.
- Microbial Life Found in Hydrocarbon Lake. the physics arXiv blog 15 April 2010.
- Schulze-Makuch, Dirk; Haque, Shirin; De Sousa Antonio, Marina Resendes; Ali, Denzil; Hosein, Riad; Song, Young C.; Yang, Jinshu; Zaikova, Elena; Beckles, Denise M.; Guinan, Edward; Lehto, Harry J.; Hallam, Steven J. (2011). "Microbial Life in a Liquid Asphalt Desert". Astrobiology. 11 (3): 241–58. arXiv:1004.2047. Bibcode:2011AsBio..11..241S. doi:10.1089/ast.2010.0488. PMID 21480792. S2CID 22078593.
- Ahmed I, Yokota A, Fujiwara T (March 2007). "A novel highly boron tolerant bacterium, Bacillus boroniphilus sp. nov., isolated from soil, that requires boron for its growth". Extremophiles. 11 (2): 217–24. doi:10.1007/s00792-006-0027-0. PMID 17072687. S2CID 2965138.
- Lollar, Garnet S.; Warr, Oliver; Telling, Jon; Osburn, Magdalena R.; Lollar, Barbara Sherwood (2019). "'Follow the Water': Hydrogeochemical Constraints on Microbial Investigations 2.4 km Below Surface at the Kidd Creek Deep Fluid and Deep Life Observatory". Geomicrobiology Journal. 36 (10): 859–72. doi:10.1080/01490451.2019.1641770. S2CID 199636268.
- World’s Oldest Groundwater Supports Life Through Water-Rock Chemistry Archived 10 September 2019 at the Wayback Machine, 29 July 2019, deepcarbon.net.
- Strange life-forms found deep in a mine point to vast 'underground Galapagos', By Corey S. Powell, 7 Sept. 2019, nbcnews.com.
- "Bioenergy and Industrial Microbiology". Idaho National Laboratory. U.S. Department of Energy. Archived from the original on 18 October 2014. Retrieved 3 February 2014.
- Anitori RP, ed. (2012). Extremophiles: Microbiology and Biotechnology. Caister Academic Press. ISBN 978-1-904455-98-1.
- Johnsborg O, Eldholm V, Håvarstein LS (December 2007). "Natural genetic transformation: prevalence, mechanisms and function". Research in Microbiology. 158 (10): 767–78. doi:10.1016/j.resmic.2007.09.004. PMID 17997281.
- Moseley BE, Setlow JK (September 1968). "Transformation in Micrococcus radiodurans and the ultraviolet sensitivity of its transforming DNA". Proceedings of the National Academy of Sciences of the United States of America. 61 (1): 176–83. Bibcode:1968PNAS...61..176M. doi:10.1073/pnas.61.1.176. PMC 285920. PMID 5303325.
- Koyama Y, Hoshino T, Tomizuka N, Furukawa K (April 1986). "Genetic transformation of the extreme thermophile Thermus thermophilus and of other Thermus spp". Journal of Bacteriology. 166 (1): 338–40. doi:10.1128/jb.166.1.338-340.1986. PMC 214599. PMID 3957870.
- Rosenshine I, Tchelet R, Mevarech M (September 1989). "The mechanism of DNA transfer in the mating system of an archaebacterium". Science. 245 (4924): 1387–89. Bibcode:1989Sci...245.1387R. doi:10.1126/science.2818746. PMID 2818746.
- Fröls S, Ajon M, Wagner M, Teichmann D, Zolghadr B, Folea M, Boekema EJ, Driessen AJ, Schleper C, Albers SV, et al. (November 2008). "UV-inducible cellular aggregation of the hyperthermophilic archaeon Sulfolobus solfataricus is mediated by pili formation". Molecular Microbiology. 70 (4): 938–52. doi:10.1111/j.1365-2958.2008.06459.x. PMID 18990182.
- Ajon M, Fröls S, van Wolferen M, Stoecker K, Teichmann D, Driessen AJ, Grogan DW, Albers SV, Schleper C, et al. (November 2011). "UV-inducible DNA exchange in hyperthermophilic archaea mediated by type IV pili" (PDF). Molecular Microbiology. 82 (4): 807–17. doi:10.1111/j.1365-2958.2011.07861.x. PMID 21999488. S2CID 42880145.
- van Wolferen M, Ajon M, Driessen AJ, Albers SV (July 2013). "How hyperthermophiles adapt to change their lives: DNA exchange in extreme conditions". Extremophiles. 17 (4): 545–63. doi:10.1007/s00792-013-0552-6. PMID 23712907. S2CID 5572901.
- Gaudin M, Gauliard E, Schouten S, Houel-Renault L, Lenormand P, Marguet E, Forterre P (February 2013). "Hyperthermophilic archaea produce membrane vesicles that can transfer DNA". Environmental Microbiology Reports. 5 (1): 109–16. doi:10.1111/j.1758-2229.2012.00348.x. PMID 23757139.
- Krupovic M, Gonnet M, Hania WB, Forterre P, Erauso G (2013). "Insights into dynamics of mobile genetic elements in hyperthermophilic environments from five new Thermococcus plasmids". PLOS ONE. 8 (1): e49044. Bibcode:2013PLoSO...849044K. doi:10.1371/journal.pone.0049044. PMC 3543421. PMID 23326305.
Further reading
- Wilson ZE, Brimble MA (January 2009). "Molecules derived from the extremes of life". Natural Product Reports. 26 (1): 44–71. doi:10.1039/b800164m. PMID 19374122.
- Rossi M, Ciaramella M, Cannio R, Pisani FM, Moracci M, Bartolucci S (July 2003). "Extremophiles 2002". Journal of Bacteriology. 185 (13): 3683–3689. doi:10.1128/JB.185.13.3683-3689.2003. PMC 161588. PMID 12813059.
- C.Michael Hogan (2010). "Extremophile". Encyclopedia of Earth, National Council of Science & the Environment, Eds. E. Monosson & C. Cleveland.
- Seckbach J, Oren A, Stan-Lotter H, eds. (2013). Polyextremophiles: life under multiple forms of stress. Dordrecht: Springer. ISBN 978-94-007-6488-0.
External links
- Extreme Environments - Science Education Resource Center
- Extremophile Research Archived 18 October 2014 at the Wayback Machine
- Eukaryotes in extreme environments
- The Research Center of Extremophiles Archived 11 January 2016 at the Wayback Machine
- DaveDarling's Encyclopedia of Astrobiology, Astronomy, and Spaceflight
- The International Society for Extremophiles
- Idaho National Laboratory Archived 18 October 2014 at the Wayback Machine
- Polyextremophile on David Darling's Encyclopedia of Astrobiology, Astronomy, and Spaceflight
- T-Limit Expedition