Pyrrocaine
Pyrrocaine is a local anesthetic drug. The cogency of pyrrocaine is equivalent to lidocaine in blocking the motor nerve and sensory. Pyrrocaine was proven to be somewhat harmless compared to lidocaine. No signs of methemoglobinemia was found while observing. It was considered unsafe for acute porphyria treatment. No evidence is found that it is profitly used now.[1]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N-(2,6-Dimethylphenyl)-2-(pyrrolidin-1-yl)acetamide | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C14H20N2O |
| Molar mass | 232.327 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
History
In the 1960s it was most of the time used as a nerve blocker dental anesthetic and dentists recommended it due to its fast commencement.[1]
Adverse effects
Pyrrocane has very similar side effects on blood pressure and heart rate compared to lidocaine.[2]
Synthesis
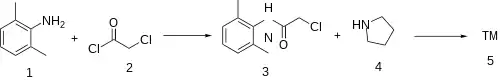
Selfsame as lidocaine, albeit interposing pyrrolidine for diethylamine.
Amide formation between 2,6-Dimethylaniline (1) and Chloroacetyl chloride (2) gives [1131-01-7] (3). Displacement of the remaining halogen by pyrrolidine (4) completed the synthesis of Pyrrocaine (5).
See also
- Procaine
- Trimecaine
- Local anesthetic
- List of local anesthetics
References
- "NCATS Inxight: Drugs". drugs.ncats.io. Retrieved 2018-08-07.
- Annals of Dentistry. New York Academy of Dentistry. 1983.
- Löfgren, Nils; Tegnér, Claës; Takman, Bertil (1957). "Studies on Local Anesthetics. XVI.". Acta Chemica Scandinavica 11: 1724–1737. doi:10.3891/acta.chem.scand.11-1724.
- Albert Schlesinger, Gordon Samuel M, U.S. Patent 2,813,861 (1957 to Endo Lab).
- Anon., GB 986993 (1965 to Graham Chemical Corp).