Respiratory epithelium
Respiratory epithelium, or airway epithelium,[1] is a type of ciliated columnar epithelium found lining most of the respiratory tract as respiratory mucosa,[2] where it serves to moisten and protect the airways. It is not present in the vocal cords of the larynx, or the oropharynx and laryngopharynx, where instead the epithelium is stratified squamous.[3] It also functions as a barrier to potential pathogens and foreign particles, preventing infection and tissue injury by the secretion of mucus and the action of mucociliary clearance.
| Respiratory epithelium | |
|---|---|
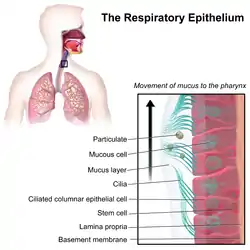 Illustration depicting the respiratory epithelium. Basal cells labelled as stem cells. | |
| Details | |
| System | Respiratory system |
| Identifiers | |
| MeSH | D020545 |
| TH | H3.05.00.0.00003 |
| Anatomical terms of microanatomy | |
| This article is part of a series on |
| Epithelia |
|---|
| Squamous epithelial cell |
| Columnar epithelial cell |
| Cuboidal epithelial cell |
| Specialised epithelia |
|
| Other |
Structure
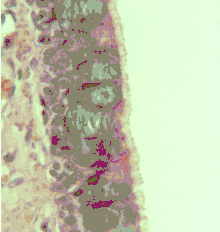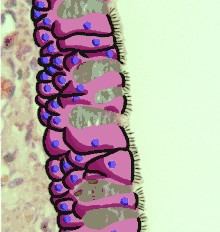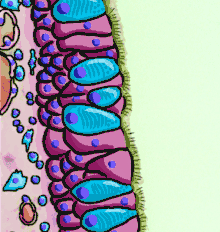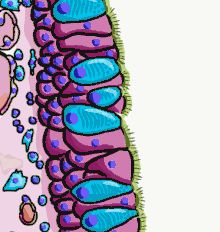

The respiratory epithelium lining the upper respiratory airways is classified as ciliated pseudostratified columnar epithelium.[4] This designation is due to the arrangement of the multiple cell types composing the respiratory epithelium. While all cells make contact with the basement membrane and are, therefore, a single layer of cells, their nuclei are not aligned in the same plane. Hence, it appears as though several layers of cells are present and the epithelium is called pseudostratified (falsely layered). The respiratory mucosa transitions to simple ciliated cuboidal epithelium and finally to simple squamous epithelium in the alveolar ducts and alveoli.[5]
Cells
The cells in the respiratory epithelium are of five main types: a) ciliated cells, b) goblet cells, c) brush cells, d) airway basal cells, and e) small granule cells (NDES)[6] Goblet cells become increasingly fewer further down the respiratory tree until they are absent in the terminal bronchioles; club cells take over their role to some extent here.[7] Another important cell type is the pulmonary neuroendocrine cell. These are innervated cells that only make up around 0.5% of the respiratory epithelial cells.[7] The ciliated cells are columnar epithelial cells with specialized ciliary modifications. The ciliated cells make up between 50 and 80 per cent of the epithelium.[8]
Between the ciliated cells are numerous microvilli, attached as tufts to brush cells sometimes referred to as pulmonary brush cells;[9] these are also known as the tuft cells of the gastrointestinal tract, or intestinal tuft cells,[10] although there is a difference between the two types: the brush cells lack the terminal web that lies under the microvilli of the tuft cells.[9] Although their function is not yet fully understood, it has been suggested that they exhibit a virulence associated clearance role, activating mucociliary clearance by releasing acetylcholine.[11]
Function
The respiratory epithelium functions to moisten and protect the airways. It acts as a physical barrier to pathogens, as well as their removal in the mechanism of mucociliary clearance.
The ciliated cells are the primary components in the mucociliary clearance mechanism. Each epithelial cell has around 200 cilia that beat constantly at a rate of between 10 and 20 times per second. The direction of their beat is targeted towards the pharynx, either upwards from the lower respiratory tract or downwards from the nasal structures.[12]
Goblet cells, so named because they are shaped like a wine goblet, are columnar epithelial cells that contain membrane-bound mucous granules and secrete mucus as part of the airway surface liquid (ASL), also known as the epithelial lining fluid, the composition of which is tightly regulated; the mucus helps maintain epithelial moisture and traps particulate material and pathogens moving through the airway. and determines how well mucociliary clearance works.[13][14]
The basal cells are small, nearly cuboidal that differentiate into the other cell types found within the epithelium. Basal cells respond to injury of the airway epithelium, migrating to cover a site denuded of differentiated epithelial cells, and subsequently differentiating to restore a healthy epithelial cell layer. The differentiated epithelial cells can also dedifferentiate into stem cells and contribute to the repairing of the barrier.[15]
Club cells carry out similar functions in the more distal airways.
Certain parts of the respiratory tract, such as the oropharynx, are also subject to the abrasive swallowing of food. To prevent the destruction of the epithelium in these areas, it changes to stratified squamous epithelium, which is better suited to the constant sloughing and abrasion. The squamous layer of the oropharynx is continuous with the esophagus.
The respiratory epithelium has a further role of immunity for the lungs - that of glucose homeostasis.[16] The glucose concentration in the airway surface liquid is held at a level of around 12 times lower than that of the blood sugar concentration.[16] The tight junctions act as a barrier that restricts the passage of glucose across the epithelium into the airway lumen. Some glucose passes through, where it diffuses into the airway surface liquid to be kept at its reduced level by pulmonary glucose transport, and metabolism.[17] However, airway inflammation decreases the effectiveness of the tight junctions making the barrier more permeable to glucose. Higher levels of glucose promote the proliferation of bacteria by providing glucose as a source for carbon for them.[16] Increased levels of glucose in the airway surface liquid is associated with respiratory diseases, and hyperglycemia.[17]
Clinical significance
Long-term irritation of the epithelial cells can cause the overproduction of mucus, known as mucus hypersecretion. Mucus hypersecretion results in the productive cough of chronic bronchitis.[18]
Pulmonary neuroendocrine cells have been associated with a range of chronic lung disorders. They are also the originating cells of small-cell lung cancer.[19]
References
- Crystal, R (September 2008). "Airway epithelial cells: current concepts and challenges". Proc Am Thorac Soc. 15 (7): 772–777. doi:10.1513/pats.200805-041HR. PMC 5820806. PMID 18757316.
- "Respiratory mucosa". meshb.nlm.nih.gov. Retrieved 26 July 2019.
- Saladin, K (2012). Anatomy & physiology: the unity of form and function (6th ed.). McGraw-Hill. pp. 857–859. ISBN 9780073378251.
- Mescher AL, "Chapter 17. The Respiratory System" (Chapter). Mescher AL: Junqueira's Basic Histology: Text & Atlas, 12e: "AccessMedicine | the Respiratory System: Introduction". Archived from the original on 2013-06-03. Retrieved 2015-02-24..
- "Bronchi, Bronchial Tree & Lungs". nih.gov. Retrieved 17 September 2019.
- Mescher, Anthony L. (2018). Junqueira's basic histology: text and atlas (Fifteenth ed.). [New York]. p. 350. ISBN 978-1-26-002618-4.
- Weinberger, Steven; Cockrill, Barbara; Mandel, Jess (2019). Principles of pulmonary medicine (Seventh ed.). Elsevier. p. 67. ISBN 9780323523714.
- Yaghi, A; Dolovich, MB (11 November 2016). "Airway Epithelial Cell Cilia and Obstructive Lung Disease". Cells. 5 (4): 40. doi:10.3390/cells5040040. PMC 5187524. PMID 27845721.
- Reid, L; Meyrick, B; Antony, VB; Chang, LY; Crapo, JD; Reynolds, HY (1 July 2005). "The mysterious pulmonary brush cell: a cell in search of a function". American Journal of Respiratory and Critical Care Medicine. 172 (1): 136–9. doi:10.1164/rccm.200502-203WS. PMC 2718446. PMID 15817800.
- Hasleton, Philip (1996). Spencer's Pathology of the Lung. McGraw-Hill. pp. 10. ISBN 0071054480.
- Perniss, Alexander; Lui, Shuya; Boonen, Brett; Zufall, Frank; Buffe, Bernd; Kummer, Wolfgang (2020-04-14). "Chemosensory Cell-Derived Acetylcholine Drives Tracheal Mucociliary Clearance in Response to Virulence-Associated Formyl Peptides". Cell Immunity. 52 (2): 683. doi:10.1016/j.immuni.2020.03.005.
- Hall, John (2011). Guyton and Hall Textbook of Medical Physiology. p. 473. ISBN 9781416045748.
- Stanke F The Contribution of the Airway Epithelial Cell to Host Defense. Mediators Inflamm. 2015;2015:463016. PMID 26185361 PMC 4491388
- U.S. EPA. Integrated Science Assessment for Oxides of Nitrogen – Health Criteria (2016 Final Report). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-15/068, 2016. Federal Register Notice Jan 28, 2016 Free download available at Report page at EPA website.
- Hiemstra, PS; McCray PB, Jr; Bals, R (April 2015). "The innate immune function of airway epithelial cells in inflammatory lung disease". The European Respiratory Journal. 45 (4): 1150–62. doi:10.1183/09031936.00141514. PMC 4719567. PMID 25700381.
- Baker, EH; Baines, DL (February 2018). "Airway Glucose Homeostasis: A New Target in the Prevention and Treatment of Pulmonary Infection". Chest. 153 (2): 507–514. doi:10.1016/j.chest.2017.05.031. PMID 28610911. S2CID 13733461.
- Garnett, JP; Baker, EH; Baines, DL (November 2012). "Sweet talk: insights into the nature and importance of glucose transport in lung epithelium". The European Respiratory Journal. 40 (5): 1269–76. doi:10.1183/09031936.00052612. PMID 22878875.
- Global Initiative for Chronic Obstructive Lung Disease - GOLD (PDF). 2018. p. 15. Retrieved 10 November 2019.
- Garg, A; Sui, P; Verheyden, JM; Young, LR; Sun, X (2019). "Consider the lung as a sensory organ: A tip from pulmonary neuroendocrine cells". Current Topics in Developmental Biology. 132: 67–89. doi:10.1016/bs.ctdb.2018.12.002. ISBN 9780128104897. PMID 30797518.
Additional images
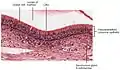 Cross-section of pseudostratified columnar epithelium
Cross-section of pseudostratified columnar epithelium Second cross-section
Second cross-section