Rhizaria
The Rhizaria are an ill-defined but species-rich supergroup of mostly unicellular[1] eukaryotes.[2] Except for the Chlorarachniophytes and three species in the genus Paulinella in the phylum Cercozoa, they are all non-photosynthethic, but many foraminifera and radiolaria have a symbiotic relationship with unicellular algae.[3] A multicellular form, Guttulinopsis vulgaris, a cellular slime mold, has also been described.[4] This group was used by Cavalier-Smith in 2002, although the term "Rhizaria" had been long used for clades within the currently recognized taxon. Being described mainly from rDNA sequences, they vary considerably in form, having no clear morphological distinctive characters (synapomorphies), but for the most part they are amoeboids with filose, reticulose, or microtubule-supported pseudopods. In the absence of an apomorphy, the group is ill-defined, and its composition has been very fluid. Some Rhizaria possess mineral exoskeleton (thecae or loricas), which is in different clades within Rhizaria made out of opal (SiO2), celestite (SrSO4), or calcite (CaCO3). It can attain sizes of more than a centimeter with some species being able to form cylindrical colonies approximately 1 cm in diameter and greater than 1 m in length. They feed by capturing and engulfing prey with the extensions of their pseudopodia; forms that are symbiotic with unicellular algae contribute significantly to the total primary production of the ocean.[5]
| Rhizaria Temporal range: | |
|---|---|
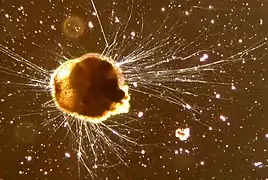 | |
| Ammonia tepida (Foraminifera) | |
| Scientific classification | |
| Domain: | Eukaryota |
| (unranked): | Diaphoretickes |
| Kingdom: | Chromista |
| Subkingdom: | Harosa |
| Infrakingdom: | Rhizaria Cavalier-Smith, 2002 |
| Phyla | |
Groups
The three main groups of Rhizaria are:[6]
- Cercozoa – various amoebae and flagellates, usually with filose pseudopods and common in soil
- Foraminifera – amoeboids with reticulose pseudopods, common as marine benthos
- Radiolaria – amoeboids with axopods, common as marine plankton
A few other groups may be included in the Cercozoa, but some trees appear closer to the Foraminifera. These are the Phytomyxea and Ascetosporea, parasites of plants and animals, respectively, and the peculiar amoeba Gromia. The different groups of Rhizaria are considered close relatives based mainly on genetic similarities, and have been regarded as an extension of the Cercozoa. The name Rhizaria for the expanded group was introduced by Cavalier-Smith in 2002,[7] who also included the centrohelids and Apusozoa.
A noteworthy order that belongs to Ascetosporea is the Mikrocytida.[8] These are parasites of oysters. This includes the causative agent of Denman Island Disease, Mikrocytos mackini a small (2−3 μm diameter) amitochondriate protistan.[9]
Evolutionary relationships
Rhizaria are part of the SAR supergroup (Stramenopiles, Alveolates, Rhizaria), a grouping that had been presaged in 1993 through a study of mitochondrial morphologies.[10] SAR is currently placed in the Diaphoretickes along with Archaeplastida, Cryptista, Haptista, and several minor clades.
Historically, many rhizarians were considered animals because of their motility and heterotrophy. However, when a simple animal-plant dichotomy was superseded by a recognition of additional kingdoms, taxonomists generally placed amoebae in the kingdom Protista. When scientists began examining the evolutionary relationships among eukaryotes in the 1970's, it became clear that the kingdom Protista was paraphyletic. Rhizaria appear to share a common ancestor with Stramenopiles and Alveolates forming part of the SAR (Stramenopiles+Alveolates+Rhizaria) super assemblage.[11] Rhizaria has been supported by molecular phylogenetic studies as a monophyletic group.[12] Biosynthesis of 24-isopropyl cholestane precursors in various rhizaria[13] suggests a relevant ecological role already during the Ediacaran.
Phylogeny
Phylogeny based on Bass et al. 2009,[14] Howe et al. 2011,[15] and Silar 2016.[16]
|
Cercozoa |
In 2019, the Cercozoa were recognized as a basal Rhizaria group, as sister of the Retaria.[17]
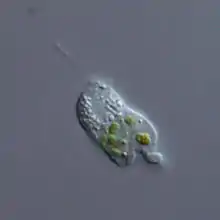 Cercomonas sp. (Cercozoa: Cercomonadida
Cercomonas sp. (Cercozoa: Cercomonadida Ebria sp. (Cercozoa: Ebridea)
Ebria sp. (Cercozoa: Ebridea)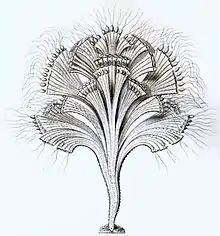 Rhipidodendron sp. (Cercozoa: Spongomonadea)
Rhipidodendron sp. (Cercozoa: Spongomonadea) Euglypha sp. (Cercozoa: Euglyphida)
Euglypha sp. (Cercozoa: Euglyphida)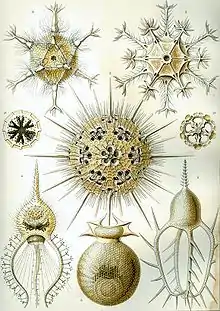 Phaeodarians (Cercozoa: Phaeodarea)
Phaeodarians (Cercozoa: Phaeodarea) Clathrulina elegans (Cercozoa: Granofilosea: Desmothoracida)
Clathrulina elegans (Cercozoa: Granofilosea: Desmothoracida) Chlorarachnion sp. (Cercozoa: (Chlorarachniophyta)
Chlorarachnion sp. (Cercozoa: (Chlorarachniophyta) Vampyrella sp. (Cercozoa: Vampyrellidae)
Vampyrella sp. (Cercozoa: Vampyrellidae)_b_083.jpg.webp)
_of_potatoes_(1914)_(14577838889).jpg.webp) Powdery scab (Cercozoa: Plasmodiophorida)
Powdery scab (Cercozoa: Plasmodiophorida) Foraminiferans (Retaria: Foraminifera)
Foraminiferans (Retaria: Foraminifera) Polycystines (Retaria: Radiolaria)
Polycystines (Retaria: Radiolaria) Acantharians (Retaria: Radiolaria)
Acantharians (Retaria: Radiolaria)
References
- Christopher Taylor (2004). "Rhizaria". Archived from the original on 2009-04-20.
- Nikolaev SI, Berney C, Fahrni JF, et al. (May 2004). "The twilight of Heliozoa and rise of Rhizaria, an emerging supergroup of amoeboid eukaryotes". Proc. Natl. Acad. Sci. U.S.A. 101 (21): 8066–71. doi:10.1073/pnas.0308602101. PMC 419558. PMID 15148395.
- Gast, Rebecca J.; Caron, David A. (2001-10-01). "Photosymbiotic associations in planktonic foraminifera and radiolaria". Hydrobiologia. 461 (1): 1–7. doi:10.1023/A:1012710909023. ISSN 1573-5117. S2CID 1387879.
- Brown; et al. (2012). "Aggregative Multicellularity Evolved Independently in the Eukaryotic Supergroup Rhizaria". Current Biology. 22 (12): 1123–1127. doi:10.1016/j.cub.2012.04.021. PMID 22608512.
- Caron, D. (2016). The rise of Rhizaria. Nature (London), 532(7600), 444–445. https://doi.org/10.1038/nature17892
- Moreira D, von der Heyden S, Bass D, López-García P, Chao E, Cavalier-Smith T (July 2007). "Global eukaryote phylogeny: Combined small- and large-subunit ribosomal DNA trees support monophyly of Rhizaria, Retaria and Excavata". Mol. Phylogenet. Evol. 44 (1): 255–66. doi:10.1016/j.ympev.2006.11.001. PMID 17174576.
- Cavalier-Smith, Thomas (2002). "The phagotrophic origin of eukaryotes and phylogenetic classification of Protozoa". International Journal of Systematic and Evolutionary Microbiology. 52 (2): 297–354. doi:10.1099/00207713-52-2-297. ISSN 1466-5026. PMID 11931142. Retrieved 2007-06-08.
- Hartikainen, H; Stentiford, GD; Bateman, KS; Berney, C; Feist, SW; Longshaw, M; Okamura, B; Stone, D; Ward, G; Wood, C; Bass, D (2014). "Mikrocytids are a broadly distributed and divergent radiation of parasites in aquatic invertebrates" (PDF). Curr Biol. 24 (7): 807–12. doi:10.1016/j.cub.2014.02.033. PMID 24656829. S2CID 17180719.
- Hine, Pm; Bower, Sm; Meyer, Gr; Cochennec-Laureau, N; Berthe, Fcj (2001). "Ultrastructure of Mikrocytos mackini, the cause of Denman Island disease in oysters Crassostrea spp. and Ostrea spp. in British Columbia, Canada". Diseases of Aquatic Organisms. 45 (3): 215–227. doi:10.3354/dao045215. ISSN 0177-5103. PMID 11558731.
- Seravin LN. Osnovnye tipy i formy tonkogo stroeniia krist mitokhondriĭ: stepen' ikh évoliutsionnoĭ stabil'nosti (sposobnost' k morfologicheskim transformatsiiam) [The basic types and forms of the fine structure of mitochondrial cristae: the degree of their evolutionary stability (capacity for morphological transformations)]. Tsitologiia. 1993;35(4):3-34. Russian. PMID 8328023.
- Burki, F; Shalchian-Tabrizi, K; Minge, M; Skjaeveland, A; Nikolaev, SI; Jakobsen, KS; Pawlowski, J (2007). Butler, Geraldine (ed.). "Phylogenomics Reshuffles the Eukaryotic Supergroups". PLoS ONE. 2 (8): e790–. Bibcode:2007PLoSO...2..790B. doi:10.1371/journal.pone.0000790. PMC 1949142. PMID 17726520.
- Burki, Fabien; Shalchian-Tabrizi, Kamran; Pawlowski, Jan (August 23, 2008). "Phylogenomics reveals a new 'megagroup' including most photosynthetic eukaryotes". Biology Letters. 4 (4): 366–9. doi:10.1098/rsbl.2008.0224. PMC 2610160. PMID 18522922.
- Hallmann, Christian; Stuhr, Marleen; Kucera, Michal; Zonneveld, Karin; Bobrovskiy, Ilya; Bowser, Samuel S.; Pawlowski, Jan; Deckker, Patrick De; Nowack, Eva C. M. (2019-03-04). "Putative sponge biomarkers in unicellular Rhizaria question an early rise of animals". Nature Ecology & Evolution. 3 (4): 577–581. doi:10.1038/s41559-019-0806-5. ISSN 2397-334X. PMID 30833757. S2CID 71148672.
- Bass D, Chao EE, Nikolaev S, et al. (February 2009). "Phylogeny of Novel Naked Filose and Reticulose Cercozoa: Granofilosea cl. n. and Proteomyxidea Revised". Protist. 160 (1): 75–109. doi:10.1016/j.protis.2008.07.002. PMID 18952499.
- Howe; et al. (2011), "Novel Cultured Protists Identify Deep-branching Environmental DNA Clades of Cercozoa: New Genera Tremula, Micrometopion, Minimassisteria, Nudifila, Peregrinia", Protist, 162 (2): 332–372, doi:10.1016/j.protis.2010.10.002, PMID 21295519
- Silar, Philippe (2016), "Protistes Eucaryotes: Origine, Evolution et Biologie des Microbes Eucaryotes", HAL Archives-ouvertes: 1–462
- Irwin, Nicholas A.T.; Tikhonenkov, Denis V.; Hehenberger, Elisabeth; Mylnikov, Alexander P.; Burki, Fabien; Keeling, Patrick J. (2019-01-01). "Phylogenomics supports the monophyly of the Cercozoa". Molecular Phylogenetics and Evolution. 130: 416–423. doi:10.1016/j.ympev.2018.09.004. ISSN 1055-7903. PMID 30318266. S2CID 52982396.