Secondary sex characteristic
Secondary sex characteristics are features that appear during puberty in humans, and at sexual maturity in other animals.[1][2] These characteristics are particularly evident in the sexually dimorphic phenotypic traits that distinguish the sexes of a species,[3] but unlike the sex organs (primary sex characteristics), are not directly part of the reproductive system.[4] Secondary sex characteristics are believed to be the product of sexual selection for traits which display fitness, giving an organism an advantage over its rivals in courtship and in aggressive interactions.[5]

Secondary sex characteristics include, for example, the manes of male lions,[2] the bright facial and rump coloration of male mandrills, and horns in many goats and antelopes. These characteristics are believed to be produced by a positive feedback loop known as the Fisherian runaway produced by the secondary characteristic in one sex and the desire for that characteristic in the other sex. Male birds and fish of many species have brighter coloration or other external ornaments. Differences in size between sexes are also considered secondary sexual characteristics.
In humans, visible secondary sex characteristics include enlarged breasts and widened hips of females, facial hair and Adam's apples on males, and pubic hair on both.[4][6]
Secondary Sex Characteristics vs. Primary Sex Characteristics
The reproductive organs in male or female organisms are usually identifiable at birth and are ascribed as the Primary Somatic Sex Characteristics. In the male, this would be the penis, scrotum, and the ability to produce sperm that will help form a zygote. In the female, this would be the uterus, vagina, fallopian tubes, clitoris, cervix, and the ability to have offspring. The primary sex organs are different from the secondary sex organs because they produce gametes, which is a mature haploid germ cell male or female which will unite with another of the opposite sex during sexual reproduction to form a zygote. The secondary sex characteristics differ in that they will not be identifiable at birth, they will develop over time as the subject matures and becomes sexually active. Those characteristics are breast in females and greater muscle mass in males. Secondary sexual characteristics have an evolutionary purpose: increase the chance of breeding.[7] In the animal kingdom, an extraordinary diversity of structures exists that cannot be explained by natural selection (Darwin 1871).[8]
Evolutionary roots

In The Descent of Man and Selection in Relation to Sex, Charles Darwin hypothesized that sexual selection, or competition within a species for mates, can explain observed differences between sexes in many species.[9]
Ronald Fisher, the English biologist, developed a number of ideas concerning secondary characteristics in his 1930 book The Genetical Theory of Natural Selection, including the concept of Fisherian runaway which postulates that the desire for a characteristic in females combined with that characteristic in males can create a positive feedback loop or runaway where the feature becomes hugely amplified. The 1975 handicap principle extends this idea, stating that a peacock's tail, for instance, displays fitness by being a useless impediment that is very hard to fake. Another of Fisher's ideas is the sexy son hypothesis, whereby females will desire to have sons that possess the characteristic that they find sexually attractive in order to maximize the number of grandchildren they produce.[10] An alternative hypothesis is that some of the genes that enable males to develop impressive ornaments or fighting ability may be correlated with fitness markers such as disease resistance or a more efficient metabolism. This idea is known as the good genes hypothesis.
In non-human animals
Secondary sex characteristics in non-human animals include manes of male lions[2] and long feathers of male peafowl, the tusks of male narwhals, enlarged proboscises in male elephant seals and proboscis monkeys, the bright facial and rump coloration of male mandrills, and horns in many goats and antelopes. [11]
Biologists today distinguish between "male-to-male combat" and "mate choice", usually female choice of male mates. Sexual characteristics due to combat are such things as antlers, horns, and greater size. Characteristics due to mate choice, often referred to as ornaments, include brighter plumage, coloration, and other features that have no immediate purpose for survival or combat.[12]
Male jumping spiders have visual patches of UV reflectance, which are ornamentations used to attract females.[13]
In humans
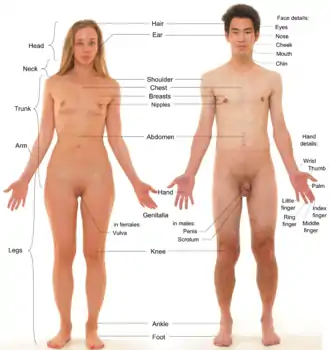
Sexual differentiation begins during gestation, when the gonads are formed. The general structure and shape of the body and face, as well as sex hormone levels, are similar in preadolescent boys and girls. As puberty begins and sex hormone levels rise, differences appear, though some changes are similar in males and females. Male levels of testosterone directly induce the growth of the genitals, and indirectly (via dihydrotestosterone (DHT)) the prostate. Estradiol and other hormones cause breasts to develop in females. However, fetal or neonatal androgens may modulate later breast development by reducing the capacity of breast tissue to respond to later estrogen.[14][15][16]
Underarm hair and pubic hair are usually considered secondary sex characteristics,[6] but they may also be considered non-secondary sex characteristics because they are features of both sexes following puberty.[17]
Females
In females, breasts are a manifestation of higher levels of estrogen; estrogen also widens the pelvis and increases the amount of body fat in hips, thighs, buttocks, and breasts.[4][6] Estrogen also induces growth of the uterus, proliferation of the endometrium, and menstruation.[4] Female secondary sex characteristics include:
- Enlargement of breasts and erection of nipples.[4][6]
- Growth of body hair, most prominently underarm and pubic hair.[2][4][6]
- Widening of hips;[4][6] lower waist to hip ratio than adult males.[18]
- Elbows that hyperextend 5–8° more than male adults.[19]
- Upper arms approximately 2 cm longer, on average, for a given height.[20]
- Labia minora, the inner lips of the vulva, may grow more prominent and undergo changes in color with the increased stimulation related to higher levels of estrogen.[21]
Males
The increased secretion of testosterone from the testes during puberty causes the male secondary sexual characteristics to be manifested.[22] In males, testosterone directly increases size and mass of muscles, vocal cords, and bones, deepening the voice, and changing the shape of the face and skeleton.[4] Converted into DHT in the skin, it accelerates growth of androgen-responsive facial and body hair but may slow and eventually stop the growth of head hair. Taller stature is largely a result of later puberty and slower epiphyseal fusion. Male secondary sex characteristics include:
- Growth of body hair, including underarm, abdominal, chest hair and pubic hair.[2][4]
- Growth of facial hair.[4]
- Enlargement of larynx (Adam's apple) and deepening of voice.[4][23]
- Increased stature; adult males are taller than adult females, on average.[4]
- Heavier skull and bone structure.[4]
- Increased muscle mass and strength.[4]
- Broadening of shoulders and chest; shoulders wider than hips.[24]
- Increased secretions of oil and sweat glands.[23]
References
- Melmed S, Polonsky KS, Larsen PR, Kronenberg HM (2011). Williams Textbook of Endocrinology E-Book. Elsevier Health Sciences. p. 1054. ISBN 978-1437736007.
- Pack PE (2016). CliffsNotes AP Biology (5th ed.). Houghton Mifflin Harcourt. p. 219. ISBN 978-0544784178.
- Norris DO, Lopez KH (2010). Hormones and Reproduction of Vertebrates, Volume 1. Academic Press. p. 87. ISBN 978-0080958095.
- Bjorklund DF, Blasi CH (2011). Child and Adolescent Development: An Integrated Approach. Cengage Learning. pp. 152–153. ISBN 978-1133168379.
- Campbell B (2017). Human Evolution: An Introduction to Man's Adaptations. Routledge. pp. 392–393. ISBN 978-1351514415.
- Rizzo DC (2015). Fundamentals of Anatomy and Physiology. Cengage Learning. pp. 483–484. ISBN 978-1305445420.
- https://study.com/learn/lesson/secondary-sex-characteristics-traits-examples.html
- Darwin, C. (1871). The descent of man, and selection in relation to sex (1st ed.). London: John Murray.Emlen, D. J. (2008). The evolution of animal weapons.
- "Sexual selection". Darwin Correspondence Project. University of Cambridge. Retrieved 1 September 2022.
- Weatherhead PJ, Robertson RJ (Feb 1979). "Offspring quality and the polygyny threshold: "The sexy son hypothesis"". American Naturalist. 113 (2): 201–208. doi:10.1086/283379. S2CID 85283084.
- "Primary and Secondary Sex Characteristics". Retrieved 14 August 2020.
- ^ Ronald Fisher in a letter to Charles Galton Darwin, 22 November 1932, cited in Fisher, R. A., Bennett, J. H. 1999. The genetical theory of natural selection: A complete variorum edition, Oxford University Press, Oxford, p. 308
- Lim, Matthew L. M., and Daiqin Li. "Courtship and Male-Male Agonistic Behaviour of Comsophasis Umbratica Simon, an Ornate Jumping Spider (Araneae: Salticidae)." The Raffles Bulletin of Zoology (2004): 52(2): 435-448. National University of Singapore. 20 September 2015
- G. Raspé (22 October 2013). Hormones and Embryonic Development: Advances in the Biosciences. Elsevier Science. pp. 32–. ISBN 978-1-4831-5171-7.
The first genetic male child with a defect in 3β-hydroxy-Δ5-steroid oxidoreductase to have reached puberty has been reported to have a high level of 3β-hydroxy-A5 steroid excretion, hypospadias at birth, salt-wasting, and a history of two siblings with congenital adrenal hyperplasia and ambiguous genitalia [33]. Although at puberty he has signs of virilization, he has developed pronounced gynecomastia. Thus, this boy demonstrates that breast development may occur in postpubertal males if the programing of the pubertal sex differentiation of the mammary gland anlagen is disturbed by an enzyme defect which causes a failure of fetal testicular testosterone production. This observation is completely consistent with the findings in the experimental models [11, 32].
- Neumann F, Elger W (March 1967). "Steroidal stimulation of mammary glands in prenatally feminized male rats". Eur. J. Pharmacol. 1 (2): 120–3. doi:10.1016/0014-2999(67)90048-9. PMID 6070056.
When administered to gravid rats during pregnancy an anti-androgenic steroid [cyproterone acetate] induced development of nipples in male fetuses. These nipples and associated glandular tissues develop after birth as in normal female animals. Progestin-estrogen treatment of adult, castrated feminized males produced stimulation of the glandular tissue similar to that seen after treatment of castrated female animals. In castrated male rats this treatment produces little glandular proliferation. It is concluded that androgens normally prevent the development of nipples and extensive formation of mammary tissue in male fetuses.
- Rajendran KG, Shah PN, Dubey AK, Bagli NP (1977). "Mammary gland differentiation in adult male rat--effect of prenatal exposure to cyproterone acetate". Endocr Res Commun. 4 (5): 267–74. doi:10.3109/07435807709052946. PMID 608453.
Breast tissue of adult male Holtzman rats exposed to cyproterone acetate during embryonic differentiation showed presence of specific estradiol receptor proteins and C-19 steroid aromatase. We reported similar findings in gynecomastia in man. It is therefore proposed that gynecomastia probably results from failure of adequate testosterone action on the breast primordia during embryonic differentiation.
- Sherwood L (2011). Fundamentals of Human Physiology. Cengage Learning. p. 578. ISBN 978-0840062253.
- Buss, David (March 15, 2019). Evolutionary Psychology: The New Science of the Mind (Sixth ed.). Routledge. p. 290. ISBN 9780429590061.
- Amis AA, Miller JH (Dec 1982). "The elbow". Clinics in Rheumatic Diseases. 8 (3): 571–93. doi:10.1016/S0307-742X(21)00409-4. PMID 7184689.
- Manwatching: A Field Guide to Human Behaviour, 1977, Desmond Morris
- Lloyd, Jillian (May 2005). "Female genital appearance: 'normality' unfolds". British Journal of Obstetrics and Gynaecology. 12 (5): 643–646. CiteSeerX 10.1.1.585.1427. doi:10.1111/j.1471-0528.2004.00517.x. PMID 15842291. S2CID 17818072.
- Van de Graaff & Fox 1989, p. 933-4.
- Sexual reproduction Archived 2009-02-08 at the Wayback Machine
- "Secondary Characteristics". hu-berlin.de. Archived from the original on 2011-09-27.