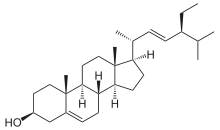
Stigmasterol
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Stigmasta-5,22-dien-3β-ol | |
| Preferred IUPAC name
(1R,3aS,3bS,7S,9aR,9bS,11aR)-1-[(2R,3E,5S)-5-Ethyl-6-methylhept-3-en-2-yl]-9a,11a-dimethyl-2,3,3a,3b,4,6,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-cyclopenta[a]phenanthren-7-ol | |
| Other names
Stigmasterin; Wulzen anti-stiffness factor | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.001.348 |
PubChem CID |
|
| UNII | |
InChI
| |
SMILES
| |
| Properties | |
Chemical formula |
C29H48O |
| Molar mass | 412.702 g·mol−1 |
| Appearance | White solid[1] |
| Melting point | 160 to 164 °C (320 to 327 °F; 433 to 437 K)[1] |
Solubility in water |
Insoluble |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |
Stigmasterol – a plant sterol (phytosterol) – is among the most abundant of plant sterols, having a major function to maintain the structure and physiology of cell membranes.[2] In the European Union, it is a food additive listed with E number E499, and may be used in food manufacturing to increase the phytosterol content, potentially lowering the levels of LDL cholesterol.[3]
Discovery
Once called Wulzen factor in the mid-20th century, stigmasterol was discovered by the University of California physiologist Rosalind Wulzen (born 1886).[4]
Natural occurrences
Stigmasterol is an unsaturated phytosterol occurring in the plant fats or oils of numerous plants,[2] such as soybean, calabar bean, and rape seed, and in herbs used in herbalism practices, including the Chinese herbs Ophiopogon japonicus (Mai men dong), in Mirabilis jalapa.[5]
Stigmasterol is a constituent of various vegetables, legumes, nuts, seeds, and unpasteurized milk. Pasteurization will inactivate stigmasterol. Edible oils contains higher amount than vegetables.[6]
Uses
Stigmasterol is a food additive in manufactured food products in the United Kingdom and European Union.[7] It was introduced as a precursor by Percy Lavon Julian for industrial large-scale manufacture of semisynthetic progesterone,[8][9][10] a valuable human hormone that plays an important physiological role in the regulatory and tissue rebuilding mechanisms related to estrogen effects, as well as acting as an intermediate in the biosynthesis of androgens, estrogens, and corticoids. It is also used as the precursor of vitamin D3.[11]
The Upjohn company used stigmasterol as the starting raw material for commercial synthesis of cortisone in 1959.[12][13]
Research
As one of the major phytosterols, stigmasterol is included among sterol compounds in the diet having potential to reduce the risk of cardiovascular diseases.[2] Consumption of 2 grams per day of plant sterols is associated with a reduction in blood LDL cholesterol of 8–10%, possibly lowering cardiovascular disease risk.[3] As a factor in cellular processes of plants, stigmasterol may have roles in plant stress responses, metabolism, and enzymes involved in biosynthesis of plant cell membranes.[2] Stigmasterol has also been shown to exert anti-angiogenic and anti-cancer effects via the downregulation of TNF-alpha and VEGFR-2.[14]
Potential precursor of boldenone
Being a steroid, stigmasterol is precursor of anabolic steroid boldenone. Boldenone undecylenate is commonly used in veterinary medicine to induce growth in cattle, but it is also one of the most commonly abused anabolic steroids in sports. This led to suspicion that some athletes testing positive for boldenone didn't consume the steroid itself, but rather consumed foods rich in stigmasterol; this turned out not to be the case.[15][16][17]
See also
- Charantin, a stigmasteryl glucoside found in the bitter melon plant.
- Stigmastanol, a closely related phytosterol
- Sitosterol, a commonly occurring phytosterol
References
- Stigmasterol, ChemicalLand21.com
- Ferrer A, Altabella T, Arró M, Boronat A (July 2017). "Emerging roles for conjugated sterols in plants". Progress in Lipid Research. 67: 27–37. doi:10.1016/j.plipres.2017.06.002. PMID 28666916.
- Cabral CE, Klein MR (November 2017). "Phytosterols in the Treatment of Hypercholesterolemia and Prevention of Cardiovascular Diseases". Arquivos Brasileiros de Cardiologia. 109 (5): 475–482. doi:10.5935/abc.20170158. PMC 5729784. PMID 29267628.
- "Rosalind Wulzen (b. 1886)". Archives, Manuscripts and Photographs catalog. Smithsonian Institution. Retrieved 14 October 2015.
- Siddiqui S, Siddiqui BS, Adil Q, Begum S (1990). "Constituents of Mirabilis jalapa". Fitoterapia. 61 (5): 471.
- Han JH, Yang YX, Feng MY (December 2008). "Contents of phytosterols in vegetables and fruits commonly consumed in China". Biomedical and Environmental Sciences. 21 (6): 449–53. doi:10.1016/S0895-3988(09)60001-5. PMID 19263798.
- "EU-approved additives and E Numbers". Food Standards Agency, UK. 1 March 2018. Retrieved 21 February 2019.
- Sundararaman P, Djerassi C (October 1977). "A convenient synthesis of progesterone from stigmasterol". The Journal of Organic Chemistry. 42 (22): 3633–4. doi:10.1021/jo00442a044. PMID 915584.
- "Nova Transcripts: Forgotten Genius". PBS.org. 6 February 2007.
- "Giants of the Past". lipidlibrary.aocs.org. Archived from the original on 15 April 2012.
- Kametani T, Furuyama H (1987). "Synthesis of vitamin D3 and related compounds". Medicinal Research Reviews. 7 (2): 147–71. doi:10.1002/med.2610070202. PMID 3033409. S2CID 20538461.
- Hogg JA (December 1992). "Steroids, the steroid community, and Upjohn in perspective: a profile of innovation". Steroids. 57 (12): 593–616. doi:10.1016/0039-128X(92)90013-Y. PMID 1481225. S2CID 21779154.
- Soy Infocenter (2009). History of Soybean and Soyfoods in Mexico and Central America (1877-2009). ISBN 9781928914211.
- Kangsamaksin T, Chaithongyot S, Wootthichairangsan C, Hanchaina R, Tangshewinsirikul C, Svasti J (12 December 2017). Ahmad A (ed.). "Lupeol and stigmasterol suppress tumor angiogenesis and inhibit cholangiocarcinoma growth in mice via downregulation of tumor necrosis factor-α". PLOS ONE. 12 (12): e0189628. Bibcode:2017PLoSO..1289628K. doi:10.1371/journal.pone.0189628. PMC 5726636. PMID 29232409.
- Gallina G, Ferretti G, Merlanti R, Civitareale C, Capolongo F, Draisci R, Montesissa C (October 2007). "Boldenone, boldione, and milk replacers in the diet of veal calves: the effects of phytosterol content on the urinary excretion of boldenone metabolites". Journal of Agricultural and Food Chemistry. 55 (20): 8275–83. doi:10.1021/jf071097c. PMID 17844992.
- Ros MM, Sterk SS, Verhagen H, Stalenhoef AF, de Jong N (July 2007). "Phytosterol consumption and the anabolic steroid boldenone in humans: a hypothesis piloted" (PDF). Food Additives and Contaminants. 24 (7): 679–84. doi:10.1080/02652030701216727. PMID 17613052. S2CID 38614535.
- Draisci R, Merlanti R, Ferretti G, Fantozzi L, Ferranti C, Capolongo F, Segato S, Montesissa C (March 2007). "Excretion profile of boldenone in urine of veal calves fed two different milk replacers". Analytica Chimica Acta. 586 (1–2): 171–6. doi:10.1016/j.aca.2007.01.026. PMID 17386709.