Tapinarof
Tapinarof, also known as benvitimod and sold under the brand name Vtama, is a medication used for the treatment of plaque psoriasis.[1] The medication is applied to the skin.[1] Besides its use in medicine, tapinarof is a naturally occurring compound found in bacterial symbionts of nematodes which has antibiotic properties.[2][3]
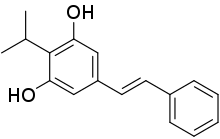 | |
| Clinical data | |
|---|---|
| Trade names | Vtama |
| Other names | Benvitimod; GSK-2894512; (E)-3,5-Dihydroxy-4-isopropyl-trans-stilbene; 3,5-Dihydroxy-4-isopropylstilbene |
| License data | |
| Routes of administration | Topical |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C17H18O2 |
| Molar mass | 254.329 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
The medication acts as an aryl hydrocarbon receptor agonist.[1][4]
Tapinarof was approved for medical use in the United States in May 2022.[1][5][6][7]
Society and culture
Names
Tapinarof is the International Nonproprietary Name (INN).[8]
Natural occurrence
_Poinar%252C_1975.jpg.webp)
Tapinarof, also known as benvitimod, is a bacterial stilbenoid produced in Photorhabdus bacterial symbionts of Heterorhabditis nematodes. It is a product of an alternative ketosynthase-directed stilbenoid biosynthesis pathway. It is derived from the condensation of two β-ketoacyl thioesters.[2] It is produced by the Photorhabdus luminescens bacterial symbiont species of the entomopathogenic nematode, Heterorhabditis megidis. Experiments with infected larvae of Galleria mellonella, the wax moth, support the hypothesis that the compound has antibiotic properties that help minimize competition from other microorganisms and prevents the putrefaction of the nematode-infected insect cadaver.[3]
See also
- Pinosylvin, a molecule produced in pines that does not bear the isopropyl alkylation.
References
- "Vtama- tapinarof cream". DailyMed. 23 May 2022. Archived from the original on 3 July 2022. Retrieved 19 June 2022.
- Joyce SA, Brachmann AO, Glazer I, Lango L, Schwär G, Clarke DJ, Bode HB (2008). "Bacterial biosynthesis of a multipotent stilbene". Angewandte Chemie. 47 (10): 1942–1945. CiteSeerX 10.1.1.603.247. doi:10.1002/anie.200705148. PMID 18236486.
- Hu K, Webster JM (August 2000). "Antibiotic production in relation to bacterial growth and nematode development in Photorhabdus--Heterorhabditis infected Galleria mellonella larvae". FEMS Microbiology Letters. 189 (2): 219–223. doi:10.1111/j.1574-6968.2000.tb09234.x. PMID 10930742.
- Bissonnette R, Stein Gold L, Rubenstein DS, Tallman AM, Armstrong A (April 2021). "Tapinarof in the treatment of psoriasis: A review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent". Journal of the American Academy of Dermatology. 84 (4): 1059–1067. doi:10.1016/j.jaad.2020.10.085. PMID 33157177.
- "Vtama: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 25 May 2022. Retrieved 24 May 2022.
- "Vtama (Tapinarof) FDA Approval History".
- "FDA Approves Dermavant's Vtama (tapinarof) cream, 1% for the Treatment of Plaque Psoriasis in Adults: First Topical Novel Chemical Entity Launched for Psoriasis in the U.S. in 25 Years". Dermavant Sciences (Press release). 24 May 2022. Archived from the original on 24 May 2022. Retrieved 24 May 2022.
- World Health Organization (2017). "International nonproprietary names for pharmaceutical substances (INN): recommended INN: list 78". WHO Drug Information. 31 (3). hdl:10665/330961.
External links
- "Tapinarof". Drug Information Portal. U.S. National Library of Medicine.