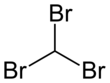
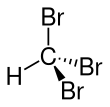
Bromoform
Bromoform (CHBr3) is a brominated organic solvent, colorless liquid at room temperature, with a high refractive index, very high density, and sweet odor is similar to that of chloroform. It is one of the four haloforms, the others being fluoroform, chloroform, and iodoform. Bromoform can be prepared by the haloform reaction using acetone and sodium hypobromite, by the electrolysis of potassium bromide in ethanol, or by treating chloroform with aluminium bromide. Currently its main use is as a laboratory reagent.
| |||
 | |||
 A bottle of bromoform with some in the adjacent beaker | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Tribromomethane[1] | |||
| Other names | |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
| Abbreviations |
| ||
Beilstein Reference |
1731048 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.000.777 | ||
| EC Number |
| ||
Gmelin Reference |
49500 | ||
| KEGG | |||
| MeSH | bromoform | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2515 | ||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
CHBr3 | ||
| Molar mass | 252.731 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Density | 2.89 g mL−1 | ||
| Melting point | −4 to 16 °C; 25 to 61 °F; 269 to 289 K | ||
| Boiling point | 147 to 151 °C; 296 to 304 °F; 420 to 424 K | ||
Solubility in water |
3.2 g L−1 (at 30 °C) | ||
| log P | 2.435 | ||
| Vapor pressure | 670 Pa (at 20.0 °C) | ||
Henry's law constant (kH) |
17 μmol Pa−1 kg−1 | ||
| Acidity (pKa) | 13.7 | ||
Magnetic susceptibility (χ) |
-82.60·10−6 cm3/mol | ||
Refractive index (nD) |
1.595 | ||
| Thermochemistry | |||
Heat capacity (C) |
130.5 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
6.1–12.7 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) |
−549.1–−542.5 kJ mol−1 | ||
| Hazards | |||
| GHS labelling: | |||
Pictograms |
  | ||
Signal word |
Danger | ||
Hazard statements |
H302, H315, H319, H331, H411 | ||
Precautionary statements |
P261, P273, P305+P351+P338, P311 | ||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
933.0 mg kg−1 (oral, rat) | ||
LDLo (lowest published) |
1400 mg/kg (mouse, oral) 1147 mg/kg (rat, oral)[3] | ||
LC50 (median concentration) |
1151 ppm (mammal)[3] | ||
LCLo (lowest published) |
4282 ppm (rat, 4 hr) 7000 ppm (dog, 1 hr)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 0.5 ppm (5 mg/m3) [skin][2] | ||
REL (Recommended) |
TWA 0.5 ppm (5 mg/m3) [skin][2] | ||
IDLH (Immediate danger) |
850 ppm[2] | ||
| Related compounds | |||
Related alkanes |
| ||
| Supplementary data page | |||
| Bromoform (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||
Structure
The molecule adopts tetrahedral molecular geometry with C3v symmetry.
Uses
Only small quantities of bromoform are currently produced industrially in the United States. In the past, it was used as a solvent, sedative and flame retardant, but now it is mainly used as a laboratory reagent, for example as an extraction solvent.
Bromoform also has medical uses; injections of bromoform are sometimes used instead of epinephrine to treat severe asthma cases.
Bromoform's high density makes it useful for separation of minerals by density. When two samples are mixed with bromoform and then allowed to settle, the top layer will contain minerals less dense than bromoform, and the bottom layer will contain denser minerals. Slightly less dense minerals can be separated in the same way by mixing the bromoform with a small amount of a less dense and miscible solvent.
Bromoform is known as an inhibitor of methanogenesis and is a common component of seaweed. Following research by CSIRO and its spin-off FutureFeed, several companies are now growing seaweed, in particular from the genus Asparagopsis, to use as a feed additive for livestock to reduce methane emissions from ruminants.[4]
Environment and Toxicology
Natural production of bromoform by phytoplankton and seaweeds in the ocean is thought to be its predominant source in the environment.[5] However, locally significant amounts of bromoform enter the environment formed as disinfection byproducts known as trihalomethanes when chlorine is added to drinking water to kill bacteria. It is somewhat soluble in water and readily evaporates into the air. Bromoform is the main trihalomethane produced in beachfront salt water swimming pools with concentrations as high as 1.2 ppm (parts per million). Concentrations in freshwater pools are 1000 times lower.[6] Occupational skin exposure limits are set at 0.5 ppm.[7]
The substance may be hazardous to the environment, and special attention should be given to aquatic organisms. Its volatility and environmental persistence makes bromoform's release, either as liquid or vapor, strongly inadvisable.
Bromoform can be absorbed into the body by inhalation and through the skin. The substance is irritating to the respiratory tract, the eyes, and the skin, and may cause effects on the central nervous system and liver, resulting in impaired functions. It is soluble in about 800 parts water and is miscible with alcohol, benzene, chloroform, ether, petroleum ether, acetone, and oils. Its LD50 is 7.2 mmol/kg in mice, or 1.8g/kg. The International Agency for Research on Cancer (IARC) concluded that bromoform is not classifiable as to human carcinogenicity. The EPA classified bromoform as a probable human carcinogen.[8][9]
References
- Betterton E. A.; Arnold R. G.; Kuhler R. J.; Santo G. A. (June 2005). "Reductive dehalogenation of bromoform in aqueous solution". Environ. Health Perspect. Brogan &. 103: 89–91(3). doi:10.2307/3432487. JSTOR 3432487. PMC 1519304. PMID 8565919. PDF
- U.S. Department of Health and Human Services. Toxicological Profile for Bromoform and Dibromochloromethane . August 2005.
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 661. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The retained names ‘bromoform’ for HCBr3, ‘chloroform’ for HCCl3, and ‘iodoform’ for HCI3 are acceptable in general nomenclature. Preferred IUPAC names are substitutive names.
- NIOSH Pocket Guide to Chemical Hazards. "#0066". National Institute for Occupational Safety and Health (NIOSH).
- "Bromoform". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- "Is seaweed the solution to agriculture's methane problem?". Phyconomy. Retrieved 2020-11-13.
- Palmer C J and Reason C J (2009), Relationships of surface bromoform concentrations with mixed layer depth and salinity in the tropical oceans (2009), Global Biogeochemical Cycles, 23, GB2014.
- Beech AJ et al (1980) Nitrates, Chlorates and Trihalomethanes in Swimming Pool Water. Am J Public Health, 70(1), 79-82
- CDC - NIOSH Pocket Guide to Chemical Hazards
- IARC - https://monographs.iarc.fr/wp-content/uploads/2018/06/mono52-10.pdf
- ATSDR - https://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=712&tid=128
External links
- International Chemical Safety Card 0108
- NIOSH Pocket Guide to Chemical Hazards. "#0066". National Institute for Occupational Safety and Health (NIOSH).
- Entry at chemicalland21.com
- Toxicological profile for bromoform and dibromochlormethane
- Toxicity summary
- IARC Summaries & Evaluations: Vol. 62 (1991), Vol. 71 (1999)
- ChemSub Online: Bromoform