Iodoform
Iodoform (also known as triiodomethane and, inaccurately, as carbon triiodide) is the organoiodine compound with the chemical formula CHI3. A pale yellow, crystalline, volatile substance, it has a penetrating and distinctive odor (in older chemistry texts, the smell is sometimes referred to as that of hospitals, where the compound is still commonly used) and, analogous to chloroform, sweetish taste. It is occasionally used as a disinfectant.
 | |||
| |||
 | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Triiodomethane | |||
| Other names
Iodoform;[1] Carbon triiodide | |||
| Identifiers | |||
CAS Number |
|||
3D model (JSmol) |
|||
Beilstein Reference |
1697010 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.795 | ||
| EC Number |
| ||
| KEGG | |||
| MeSH | iodoform | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula |
CHI3 | ||
| Molar mass | 393.732 g·mol−1 | ||
| Appearance | Pale, light yellow, opaque crystals | ||
| Odor | Saffron-like[2] | ||
| Density | 4.008 g cm−3[2] | ||
| Melting point | 119 °C (246 °F; 392 K)[2] | ||
| Boiling point | 218 °C (424 °F; 491 K)[2] | ||
Solubility in water |
100 mg L−1[2] | ||
| Solubility in diethyl ether | 136 g L−1 | ||
| Solubility in acetone | 120 g L−1 | ||
| Solubility in ethanol | 78 g L−1 | ||
| log P | 3.118 | ||
Henry's law constant (kH) |
3.4 μmol Pa−1 kg−1 | ||
Magnetic susceptibility (χ) |
−117.1·10−6 cm3/mol | ||
| Structure | |||
Crystal structure |
Hexagonal | ||
Coordination geometry |
Tetragonal | ||
Molecular shape |
Tetrahedron | ||
| Thermochemistry | |||
Heat capacity (C) |
157.5 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH⦵298) |
180.1–182.1 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH⦵298) |
−716.9 – −718.1 kJ mol−1 | ||
| Pharmacology | |||
| D09AA13 (WHO) | |||
| Hazards | |||
| GHS labelling: | |||
Pictograms |
 | ||
Signal word |
Warning | ||
Hazard statements |
H315, H319, H335 | ||
Precautionary statements |
P261, P280, P305+P351+P338 | ||
| NFPA 704 (fire diamond) | |||
| Flash point | 204 °C (399 °F; 477 K) | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
|||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
none[4] | ||
REL (Recommended) |
0.6 ppm (10 mg/m3)[4] | ||
IDLH (Immediate danger) |
N.D.[4] | ||
| Related compounds | |||
Related haloalkanes |
| ||
Related compounds |
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references | |||

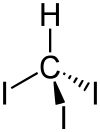
Structure
The molecule adopts tetrahedral molecular geometry with C3v symmetry.
Synthesis and reactions
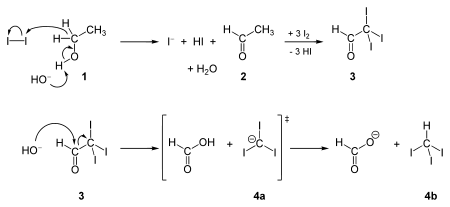
The synthesis of iodoform was first described by Georges-Simon Serullas in 1822, by reactions of iodine vapour with steam over red-hot coals, and also by reaction of potassium with ethanolic iodine in the presence of water;[5] and at much the same time independently by John Thomas Cooper.[6] It is synthesized in the haloform reaction by the reaction of iodine and sodium hydroxide with any one of these four kinds of organic compounds: a methyl ketone (CH3COR), acetaldehyde (CH3CHO), ethanol (CH3CH2OH), and certain secondary alcohols (CH3CHROH, where R is an alkyl or aryl group).
The reaction of iodine and base with methyl ketones is so reliable that the iodoform test (the appearance of a yellow precipitate) is used to probe the presence of a methyl ketone. This is also the case when testing for specific secondary alcohols containing at least one methyl group in alpha-position.
Some reagents (e.g. hydrogen iodide) convert iodoform to diiodomethane. Also conversion to carbon dioxide is possible: Iodoform reacts with aqueous silver nitrate to produce carbon monoxide. When treated with powdered elemental silver the iodoform is reduced, producing acetylene. Upon heating iodoform decomposes to produce diatomic iodine, hydrogen iodide gas, and carbon.
Natural occurrence
The angel's bonnet mushroom contains iodoform, and shows its characteristic odor.
Applications
The compound finds small-scale use as a disinfectant.[3][7] Around the beginning of the 20th century, it was used in medicine as a healing and antiseptic dressing for wounds and sores, although this use is now superseded by superior antiseptics. It is the active ingredient in many ear powders for dogs and cats, along with zinc oxide and propionic acid, which are used to prevent infection and facilitate removal of ear hair.
See also
- Iodoform reaction
- Chloroform
References
- "Front Matter". Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 661. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The retained names ‘bromoform’ for HCBr3, ‘chloroform’ for HCCl3, and ‘iodoform’ for HCI3 are acceptable in general nomenclature. Preferred IUPAC names are substitutive names.
- Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- Merck Index, 12 Edition, 5054
- NIOSH Pocket Guide to Chemical Hazards. "#0343". National Institute for Occupational Safety and Health (NIOSH).
- Surellas, Georges-Simon (1822), Notes sur l'Hydriodate de potasse et l'Acide hydriodique. -- Hydriodure de carbone; moyen d'obtenir, à l'instant, ce composé triple [Notes on the hydroiodide of potassium and on hydroiodic acid -- hydroiodide of carbon; means of obtaining instantly this compound of three elements] (in French), Metz, France: Antoine, pp. 17–20, 28–29
- James, Frank A. J. L. (2004). "Cooper, John Thomas". Oxford Dictionary of National Biography (online ed.). Oxford University Press. doi:10.1093/ref:odnb/39361. Retrieved 26 January 2012. (Subscription or UK public library membership required.)
- Lyday, Phyllis A. (2005), "Iodine and Iodine Compounds", Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, pp. 1–13, doi:10.1002/14356007.a14_381.pub2, ISBN 9783527306732
External links
- NIOSH Pocket Guide to Chemical Hazards. "#0343". National Institute for Occupational Safety and Health (NIOSH).
- MSDS at JT Baker
- A Method for the Specific Conversion of Iodoform to Carbon Dioxide
- Preparation
- . Encyclopædia Britannica. Vol. 14 (11th ed.). 1911. p. 726.