Water treatment
Water treatment is any process that improves the quality of water to make it appropriate for a specific end-use. The end use may be drinking, industrial water supply, irrigation, river flow maintenance, water recreation or many other uses, including being safely returned to the environment. Water treatment removes contaminants and undesirable components, or reduces their concentration so that the water becomes fit for its desired end-use. This treatment is crucial to human health and allows humans to benefit from both drinking and irrigation use.

Water is the most crucial compound for life on Earth, and having drinkable water is a key worldwide concern for the twenty-first century. All living things require clean, uncontaminated water as a basic requirement. Water covers more than 71 percent of the earth’s surface, but only around 1% of it is drinkable according to international standards due to various contaminations . Waste water discharge from industries, agricultural pollution, municipal wastewater, environmental and global changes are the main sources of water contamination.[1] Even trace levels of heavy metals, dyes, and microbes are hazardous to human health, aquatic systems, and the environment.[2] According to a United Nations Sustainable Development Group report from 2021, 2.3 billion people now live in water-stressed countries, and 733 million people live in high and critically water-stressed countries. [3]
To address water scarcity issues, it is becoming increasingly important to recover water from current wastewater or develop alternate water sources for human consumption. [4]
Domestic and industrial wastewater are the two types of wastewater. Domestic wastewater contains sewage, bacteria, viruses, hazardous and non-toxic organisms, sanitary outputs, rubbish, detergents, and other solid and liquid discharges from non-manufacturing processes.[5]
Drinking water treatment
Water contamination is primarily caused by the discharge of untreated wastewater from enterprises. The effluent from various enterprises, which contains varying levels of contaminants, is dumped into rivers or other water resources. The wastewater may have a high proportion of organic and inorganic contaminants at the initial discharge. Industries generate wastewater as a result of fabrication processes, processes dealing with paper and pulp, textiles, chemicals, and from various streams such as cooling towers, boilers, and production lines.[1]
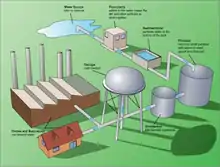
Treatment for drinking water production involves the removal of contaminants and/or inactivation of any potentially harmful microbes from raw water to produce water that is pure enough for human consumption without any short term or long term risk of any adverse health effect. In general terms, the greatest microbial risks are associated with ingestion of water that is contaminated with human or animal (including bird) faeces. Faeces can be a source of pathogenic bacteria, viruses, protozoa and helminths. The removal or destruction of microbial pathogens is essential, and commonly involves the use of reactive chemical agents such as suspended solids, to remove bacteria, algae, viruses, fungi, and minerals including iron and manganese. Research including Professor Linda Lawton's group at Robert Gordon University, Aberdeen is working to improve detection of cyanobacteria.[6] These substances continue to cause great harm to several less developed countries who do not have access to effective water purification systems.
Measures taken to ensure water quality not only relate to the treatment of the water, but to its conveyance and distribution after treatment. It is therefore common practice to keep residual disinfectants in the treated water to kill bacteriological contamination during distribution and to keep the pipes clean.
Water supplied to domestic properties such as for tap water or other uses, may be further treated before use, often using an in-line treatment process. Such treatments can include water softening or ion exchange. Many proprietary systems also claim to remove residual disinfectants and heavy metal ions.
Heavy Metals
Heavy metals in wastewater have become a serious environmental issue in recent years, owing to the high damage they pose to ecosystems and human health even at extremely low concentrations. Heavy metal pollution is a substantial environmental burden due to its flexibility, accumulation, non-biodegradability, and persistence. Its effluent is discharged into the environment by industries such as paper, Insecticides, tanneries, metal plating, mining operations, and so on. This effluent is non-biodegradable and poisonous or damaging to human physiology and other biological systems.[7]
Organic and inorganic pollutants are two types of pollutants found in wastewater, each with a different spectrum of dangerous values.
In the treatment of organic pollutants, biological, physical, and chemical methods are commonly used. However, these approaches are ineffective against inorganic pollutants such as heavy metals. Heavy metal decomposition is a serious concern due to properties such as solubility, oxidation-reduction characteristics, and complex formation.[8] Heavy metal is defined as an element with an atomic weight of between 63.5 and 200.6 and a specific gravity larger than 5.0.[9]
Heavy metals in open waters cause aquatic life to perish, oxygen deficiency, and algae blooms. When heavy metals are discharged into rivers, they are transformed into hydrated ions, which are far more hazardous than metal atoms. The enzymatical processes is disrupted by these hydrated ions, and absorption is accelerated. As a result, heavy metals must be removed in order to reduce public risk.[10]
Water Treatment Technologies
Processes

Elimination of hazardous chemicals from the water, many treatment procedures have been applied.[11] The selection of wastewater treatment systems is contingent on a number of factors: (1)The degree to which a method is necessary to raise the waste water quality to a permissible level; (2) The control method's flexibility; (3) The process's cost; and (4) The process's environmental compatibility.[4]
The processes involved in removing the contaminants include physical processes such as settling and filtration, chemical processes such as disinfection and coagulation, and biological processes such as slow sand filtration.
A combination selected from the following processes (depending on the season and contaminants and chemicals present in the raw water) is used for municipal drinking water treatment worldwide.
Chemical

Chemical approaches are used in addition to physical and biological measures to reduce the discharge of pollutants and waste water into water bodies. Different chemical procedures for the conversion into final products or the removal of pollutants are used for the safe disposal of contaminants.[4]
- Pre-chlorination for algae control and arresting biological growth.
- Aeration along with pre-chlorination for removal of dissolved iron when present with relatively small amounts of manganese.
- Disinfection for killing bacteria, viruses and other pathogens, using chlorine, ozone and ultra-violet light.
Physical
Physical techniques of water/waste water treatment rely on physical phenomena to complete the removal process, rather than biological or chemical changes.[4]
Most common physical techniques are:
- Sedimentation is one of the most important main wastewater treatment procedures. Gravity settling is a method of separating particles from a fluid. The particle in suspension remains stable in quiescent conditions due to the decrease in water velocity throughout the water treatment process, following which the particles settle by gravitational force.[12][13] For solids separation that is the removal of suspended solids trapped in the floc.
- Filtration is the technique of removing pollutants based on their particle size is known as filtration. Pollutant removal from waste water permits water to be reused for a variety of purposes. The types of filters used in the procedure differ depending on the contaminants present in the water. Particle filtration and Membrane filtration are the two main forms of waste water filtration.[14]
- Dissolved air flotation (Degasification) is the process of removing dissolved gases from a solution . The law of Henry's law states that the amount of dissolved gas in a liquid is proportionate to the partial pressure of the gas. Degasification is a low-cost method of removing carbon dioxide gas from waste water that raises the pH of the water by removing the gas.[4]
Physico-chemical
Also referred to as "Conventional" Treatment
- Coagulation for flocculation. The addition of coagulants destabilizes colloidal suspensions by neutralizing their charges, resulting in the aggregation of smaller particles during the coagulation process.[15]
- Coagulant aids, also known as polyelectrolytes – to improve coagulation and for more robust floc formation.
- Polyelectrolytes or also known in the field as polymers, usually consist of either a positive or negative charge. The nature of the polyelectrolyte used is purely based on the source water characteristics of the treatment plant.
- These will usually be used in conjunction with a primary coagulant such as ferric chloride, ferric sulfate, or alum.
Chemical Precipitation
Chemical precipitation is a common process used to reduce heavy metals concentrations in wastewater. The dissolved metal ions are transformed to an insoluble phase by a chemical interaction with a precipitant agent such as lime. In industrial applications stronger alkalis may be used to effect complete precipitation. In drinking water treatment, the common-ion effect is often used to help reduce water hardness.[16]
Flotation
Flotation uses bubble attachment to separate solids or dispersed liquids from a liquid phase.[17]
Membrane Filtration
Membrane filtration has gotten a lot of attention for inorganic effluent treatment since it can remove not only suspended solids and organic components, but also inorganic pollutants such heavy metals. For heavy metal removal, several forms of membrane filtration, such as ultrafiltration, nanofiltration, and reverse osmosis, can be used depending on the particle size that can be maintained.[18][19]
Ion Exchange
Ion exchange is a reversible ion exchange process in which an insoluble substance (resin) takes ions from an electrolytic solution and releases additional ions of the same charge in a chemically comparable amount without changing the resin's structure.[20][21]
Electrochemical Treatment Techniques [19]
- Electrodialysis (ED)
- Membrane electrolysis (ME)
- Electrochemical precipitation (EP)
Adsorption
Adsorption is a mass transfer process in which a substance is transported from the liquid phase to the surface of a solid/liquid (adsorbent) and becomes physically and chemically bonded (adsorbate). Adsorption can be classified into two forms based on the type of attraction between the adsorbate and the adsorbent: physical and chemical adsorption, commonly known as physisorption and chemisorptions.[22][23]
Activated Carbon
Activated carbons (ACs) or biological-activated carbon (BAC)[24] are effective adsorbents for a wide variety of contaminants. The adsorptive removal of color, aroma, taste, and other harmful organics and inorganics from drinking water and wastewater is one of their industrial applications.[25]
Both a high surface area and a large pore size can improve the efficiency of activated carbon. Activated carbon was utilized by a number of studies to remove heavy metals and other types of contaminants from wastewater. The cost of activated carbon is rising due to a shortage of commercial activated carbon (AC). Because of its high surface area, porosity, and flexibility, activated carbon has a lot of potential in wastewater treatment.[25]
Biological Treatment
This is the method by which dissolved and suspended organic chemical components are eliminated through biodegradation, in which an optimal amount of microorganism is given to re-enact the same natural self-purification process.[26] Through two distinct biological process, such as biological oxidation and biosynthesis, microorganisms can degrade organic materials in wastewater. Microorganisms involved in wastewater treatment produce end products such as minerals, carbon dioxide, and ammonia during the biological oxidation process. The minerals (products) remained in the wastewater and were discharged with the effluent. Microorganisms use organic materials in wastewater to generate new microbial cells with dense biomass that is eliminated by sedimentation throughout the biosynthesis process.[27]
Bioremediation
Phytoremediation, Rhizofiltration, Bioaugmentation, and Biostimulation are all biological treatment method in which microorganisms breakdown or transform hazardous contaminants in wastewater to a less toxic or non-toxic state. Both autotrophs and heterotrophs may be involved. Autotrophs can fix carbon and use inorganic chemicals in wastewater to make organic compounds such as fats, proteins, and carbohydrates. Heterotrophs feed on the soluble and emulsified organic organic substances present in the wastewater to develop and reproduce.
The sewage treatment processes of trickling filters and activated sludge both depend on maintained populations of heterotrophic organisms to do much of the work in removing contaminants. In potable water production, the hypogeal zone (the Schmutzdecke) of Slow sand filtration is a naturally developed biofilm that metabolizes organic matter, adsorbs soluble components and entraps particulates.
Technologies
Technologies for potable water and other uses are well-developed, and generalized designs are available from which treatment processes can be selected for pilot testing on the specific source water. In addition, a number of private companies provide patented technological solutions for the treatment of specific contaminants. Automation of water treatment is common in the developed world. Source water quality through the seasons, scale, and environmental impact can dictate capital costs and operating costs. End use of the treated water dictates the necessary quality monitoring technologies, and locally available skills typically dictate the level of automation adopted.
Desalination
Saline water can be treated to yield fresh water. Two main processes are used, reverse osmosis or distillation.[28] Both methods require more energy than water treatment of local surface waters, and are usually only used in coastal areas or where water such as groundwater has high salinity.[29][30]
Portable water purification
Living away from drinking water supplies often requires some form of portable water treatment process. These can vary in complexity from the simple addition of a disinfectant tablet in a hiker's water bottle through to complex multi-stage processes carried by boat or plane to disaster areas.
| Constituent | Unit processes |
| Turbidity and particles | Coagulation/ flocculation, sedimentation, granular filtration |
| Major dissolved inorganics | Softening, aeration, membranes |
| Minor dissolved inorganics | Membranes |
| Pathogens | Sedimentation, filtration, disinfection |
| Major dissolved organics | Membranes, adsorption |
Standards
Many developed countries specify standards to be applied in their own country. In Europe, this includes the European Drinking Water Directive[31] and in the United States the United States Environmental Protection Agency (EPA) establishes standards as required by the Safe Drinking Water Act. For countries without a legislative or administrative framework for such standards, the World Health Organization publishes guidelines on the standards that should be achieved.[32] China adopted its own drinking water standard GB3838-2002 (Type II) enacted by Ministry of Environmental Protection in 2002.[33]
Where drinking water quality standards do exist, most are expressed as guidelines or targets rather than requirements, and very few water standards have any legal basis or, are subject to enforcement.[34] Two exceptions are the European Drinking Water Directive and the Safe Drinking Water Act in the United States, which require legal compliance with specific standards.
Industrial water treatment

Processes
Two of the main processes of industrial water treatment are boiler water treatment and cooling water treatment. A large amount of proper water treatment can lead to the reaction of solids and bacteria within pipe work and boiler housing. Steam boilers can suffer from scale or corrosion when left untreated. Scale deposits can lead to weak and dangerous machinery, while additional fuel is required to heat the same level of water because of the rise in thermal resistance. Poor quality dirty water can become a breeding ground for bacteria such as Legionella causing a risk to public health.
Corrosion in low pressure boilers can be caused by dissolved oxygen, acidity and excessive alkalinity. Water treatment therefore should remove the dissolved oxygen and maintain the boiler water with the appropriate pH and alkalinity levels. Without effective water treatment, a cooling water system can suffer from scale formation, corrosion and fouling and may become a breeding ground for harmful bacteria. This reduces efficiency, shortens plant life and makes operations unreliable and unsafe.[35]
Boiler water treatment
Boiler water treatment is a type of industrial water treatment focused on removal or chemical modification of substances potentially damaging to the boiler. Varying types of treatment are used at different locations to avoid scale, corrosion, or foaming. External treatment of raw water supplies intended for use within a boiler is focused on removal of impurities before they reach the boiler. Internal treatment within the boiler is focused on limiting the tendency of water to dissolve the boiler, and maintaining impurities in forms least likely to cause trouble before they can be removed from the boiler in boiler blowdown.
Cooling water treatment
Water cooling is a method of heat removal from components of machinery and industrial equipment. Water may be a more efficient heat transfer fluid where air cooling is ineffective. In most occupied climates water offers the thermal conductivity advantages of a liquid with unusually high specific heat capacity and the option that of evaporative cooling. Low cost often allows rejection as waste after a single use, but recycling coolant loops may be pressurized to eliminate evaporative loss and offer greater portability and improved cleanliness. Unpressurized recycling coolant loops using evaporative cooling require a blowdown waste stream to remove impurities concentrated by evaporation. Disadvantages of water cooling systems include accelerated corrosion and maintenance requirements to prevent heat transfer reductions from biofouling or scale formation. Chemical additives to reduce these disadvantages may introduce toxicity to wastewater. Water cooling is commonly used for cooling automobile internal combustion engines and large industrial facilities such as nuclear and steam electric power plants, hydroelectric generators, petroleum refineries and chemical plants.
Chemical treatment
Chemical treatments utilizes the additive of chemicals to make industrial water suitable for use or discharge. These includes processes like chemical precipitation, chemical disinfection, Advanced oxidation process (AOP), ion exchange, and chemical neutralization.[36] AOPs are attractive in the treatment of hazardous wastewater due to its high oxidation potential and degradation performance.[37][38] In AOPs, oxidants like Fenton's reagent, Ozone or Hydrogen peroxide are introduced in the wastewater to degrade harmful substances in industrial water for discharge.
Physical treatment
Physical treatment involves the separation of solids form industrial wastewater either through Filtration or Dissolved air flotation. Filtration involves the use of Membrane or filters such as mechanical filters like sand filtration etc to achieve solid-liquid separation. Whereas for Dissolved air flotation,
pressurized air is pumped into the wastewater. The pressurized air then forms small bubbles which adhere to the suspended matter causing them to float to the surface of the water where they can be removed by a skimming device or an overflow.[39]
Biological treatment
Biological treatment is needed to treat wastewater containing biodegradable elements. It is commonly used in municipal and industrial wastewater management facilities and usually consists in adding common bacteria and other microbes, mostly environmentally friendly, to treat the water. It is a sustainable practice that has been successful for over a century.
Slow sand filters use a biological process to purify raw water to produce potable water.[40] They work by using a complex biological film that grows naturally on the surface of sand. This gelatinous biofilm called the hypogeal layer or Schmutzdecke is located in the upper few millimetres of the sand layer. The surface biofilm purifies the water as it flows through the layer, the underlying sand provides a support medium for the biological treatment layer.[41] The Schmutzdecke consists of bacteria, fungi, protozoa, rotifera and a range of aquatic insect larvae. As the biofilm ages, more algae may develop and larger aquatic organisms including bryozoa, snails and Annelid worms may be present. As water passes through the hypogeal layer, particles of matter are trapped in the mucilaginous matrix and soluble organic material is adsorbed. The contaminants are metabolised by the bacteria, fungi and protozoa.[40]
Slow sand filters are typically 1–2 metres deep, and have a hydraulic loading rate of 0.2–0.4 cubic metres per square metre per hour.[41] Filters lose their performance as the biofilm thickens and reduces the rate of flow. The filter is refurbished by removing the biofilm and a thin upper layer of sand. Water is decanted back into the filter and re-circulated to enable a new biofilm to develop. Alternatively wet harrowing involves stirring the sand and flushing the biolayer through for disposal.[41]
Physio-chemical treatment
(Also referred to as 'conventional treatment'.) Chemical flocculants are used to generate a floc in the water that traps suspended solids. Chemical polyelectrolytes are used to increase coagulation of suspended solids to improve removal.[42]
- This consist of a primary coagulant such as ferric sulfate and a coagulant aid cationic polymer being flash-mixed before it enters a Flocculation Basin.
- Once the source water being treated has been flash-mixed with a primary coagulant and a polymer, they are then put into some type of flocculation basin, where slow turning or mixing of the water, mixes the chemicals together and they can then form what is called "floc" or "flocc", which then settles out to the bottom of the floc basin.
- After the water has mixed and the floc has formed, it is then passed to the next stage which would be the settling basin. Here the process would have either tube settlers or plate settlers. The water would flow up through these tubes or plates, allowing the clear water to flow over into an effluent launder, which would then carry the "settled" water to the filters for further treatment.
- The tubes/plates in the settling stage, allow a greater surface area for the "floc" to settle on. These plates are typically at a 30–45° angle, allowing the floc particles to collect in the tubes or the plates and eventually ending up in the bottom of the settling basin.
- There is typically some sort of sludge collection system that then will collect all of the settled floc a.k.a. sludge, and pump it or transfer the waste to a decant tank or basin, where it is later disposed of.
- Once the settled water had traveled to the filters, and has made its way through the filters, it is then stored in a clearwell, where all the filtered water gets collected for additional chemical addition: pH adjuster, chlorine or UV light.
- After the appropriate contact time or kill time, the water leaves the clearwell and heads out to storage tanks or into the distribution, all the way to the customers faucet for use
Developing countries
Appropriate technology options in water treatment include both community-scale and household-scale point-of-use (POU) or self-supply designs.[43] Such designs may employ solar water disinfection methods, using solar irradiation to inactivate harmful waterborne microorganisms directly, mainly by the UV-A component of the solar spectrum, or indirectly through the presence of an oxide photocatalyst, typically supported TiO2 in its anatase or rutile phases.[44] Despite progress in SODIS technology, military surplus water treatment units like the ERDLator are still frequently used in developing countries. Newer military style Reverse Osmosis Water Purification Units (ROWPU) are portable, self-contained water treatment plants are becoming more available for public use.[45]
For waterborne disease reduction to last, water treatment programs that research and development groups start in developing countries must be sustainable by the citizens of those countries. This can ensure the efficiency of such programs after the departure of the research team, as monitoring is difficult because of the remoteness of many locations.
Energy Consumption: Water treatment plants can be significant consumers of energy. In California, more than 4% of the state's electricity consumption goes towards transporting moderate quality water over long distances, treating that water to a high standard.[46] In areas with high quality water sources which flow by gravity to the point of consumption, costs will be much lower. Much of the energy requirements are in pumping. Processes that avoid the need for pumping tend to have overall low energy demands. Those water treatment technologies that have very low energy requirements including trickling filters, slow sand filters, gravity aqueducts.
A 2021 study found that a large-scale water chlorination program in urban areas of Mexico massively reduced childhood diarrheal disease mortality rates.[47]
Drinking water regulation
New Zealand
The Water Services Act 2021 brought Taumata Arowai' into existence as the new regulator of drinking water and waste water treatment in New Zealand. Initial activities including the establishment of a national register of water suppliers and establishing a network of accredited laboratories for drinking water and waste water analysis[48]
Singapore
Singapore is a significant importer of water from neighbouring Malaysia but also has a made great efforts to reclaim as much used water as possible to enure adequate provision for the very crowded city/state. Their reclaimed water is marketed as NEWater. Singapore updated its water quality regulation in 2019 setting standards consistent with WHO recommended standards. Monitoring is undertaken by the Environmental Public Health Department of the Singaporean Government[49]
United Kingdom
In the United Kingdom regulation of water supplies is a devolved matter to the Welsh and Scottish Parliaments and the Northern Ireland Assembly.
In England and Wales there are two water industry regulatory authorities.
- Water Services Regulation Authority (Ofwat) is the economic regulator of the water sector; it protects the interests of consumers by promoting effective competition and ensuring that water companies carry out their statutory functions. Ofwat has a management board comprising a Chairman, Chief Executive and Executive and Non-Executive members. There is a staff of about 240.[50]
- The Drinking Water Inspectorate (DWI) provides independent assurance that the privatised water industry delivers safe, clean drinking water to consumers. The DWI was established in 1990 and comprises a Chief Inspector of Drinking Water and a team of about 40 people.[51] The current standards of water quality are defined in Statutory Instrument 2016 No. 614 the Water Supply (Water Quality) Regulations 2016.[52]
The functions and duties of the bodies are formally defined in the Water Industry Act 1991 (1991 c. 56) as amended by the Water Act 2003 (2003 c. 37) and the Water Act 2014 (2014 c. 21).[53]
In Scotland water quality is the responsibility of independent Drinking Water Quality Regulator (DWQR).[54]
In Northern Ireland the Drinking Water Inspectorate (DWI) regulates drinking water quality of public and private supplies.[55] The current standards of water quality are defined in the Water Supply (Water Quality) Regulations (Northern Ireland) 2017.[56]
United States
The Safe Drinking Water Act requires the U.S. Environmental Protection Agency (EPA) to set standards for drinking water quality in public water systems (entities that provide water for human consumption to at least 25 people for at least 60 days a year).[57] Enforcement of the standards is mostly carried out by state health agencies.[58] States may set standards that are more stringent than the federal standards.[59]
EPA has set standards for over 90 contaminants organized into six groups: microorganisms, disinfectants, disinfection byproducts, inorganic chemicals, organic chemicals and radionuclides.[60]
EPA also identifies and lists unregulated contaminants which may require regulation. The Contaminant Candidate List is published every five years, and EPA is required to decide whether to regulate at least five or more listed contaminants.[61]
Local drinking water utilities may apply for low interest loans, to make facility improvements, through the Drinking Water State Revolving Fund.[62]
See also
- Agricultural wastewater treatment
- Control of water pollution
- Clean Water Act
- Peak water (water supply & demand)
- Pulsed-power water treatment
- Reclaimed water
- Sewage treatment
- Wastewater treatment
- Water purification
- Water quality
- Water softening
- Water supply
- Heavy metals
- Adsorption
- Activated carbon
References
- Singh, N. B.; Nagpal, Garima; Agrawal, Sonal; Rachna (2018-08-01). "Water purification by using Adsorbents: A Review". Environmental Technology & Innovation. 11: 187–240. doi:10.1016/j.eti.2018.05.006. ISSN 2352-1864. S2CID 103693107.
- Khan, Muhammad Usman; Malik, Riffat Naseem; Muhammad, Said (2013-11-01). "Human health risk from Heavy metal via food crops consumption with wastewater irrigation practices in Pakistan". Chemosphere. 93 (10): 2230–2238. Bibcode:2013Chmsp..93.2230K. doi:10.1016/j.chemosphere.2013.07.067. ISSN 0045-6535. PMID 24075531.
- "Summary Progress Update 2021: SDG 6 — water and sanitation for all".
- Saravanan, A.; Senthil Kumar, P.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P. R.; Reshma, B. (2021-10-01). "Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development". Chemosphere. 280: 130595. Bibcode:2021Chmsp.280m0595S. doi:10.1016/j.chemosphere.2021.130595. ISSN 0045-6535. PMID 33940449.
- Tee, Pei Fang; Abdullah, Mohammad Omar; Tan, Ivy Ai Wei; Rashid, Nur Khairunnisa Abdul; Amin, Mohamed Afizal Mohamed; Nolasco-Hipolito, Cirilo; Bujang, Kopli (2016-02-01). "Review on hybrid energy systems for wastewater treatment and bio-energy production". Renewable and Sustainable Energy Reviews. 54: 235–246. doi:10.1016/j.rser.2015.10.011. ISSN 1364-0321.
- "Linda Lawton – 11th International Conference on Toxic Cyanobacteria". Retrieved 2021-06-25.
- Han, Weijiang; Fu, Fenglian; Cheng, Zihang; Tang, Bing; Wu, Shijiao (2016-01-25). "Studies on the optimum conditions using acid-washed zero-valent iron/aluminum mixtures in permeable reactive barriers for the removal of different heavy metal ions from wastewater". Journal of Hazardous Materials. 302: 437–446. doi:10.1016/j.jhazmat.2015.09.041. ISSN 0304-3894. PMID 26521089.
- Lee, Jae-chun; Pandey, Banshi Dhar (2012-01-01). "Bio-processing of solid wastes and secondary resources for metal extraction – A review". Waste Management. 32 (1): 3–18. doi:10.1016/j.wasman.2011.08.010. ISSN 0956-053X. PMID 21925857.
- Srivastava, N. K.; Majumder, C. B. (2008-02-28). "Novel biofiltration methods for the treatment of heavy metals from industrial wastewater". Journal of Hazardous Materials. 151 (1): 1–8. doi:10.1016/j.jhazmat.2007.09.101. ISSN 0304-3894. PMID 17997034.
- Carolin, C. Femina; Kumar, P. Senthil; Saravanan, A.; Joshiba, G. Janet; Naushad, Mu. (2017-06-01). "Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review". Journal of Environmental Chemical Engineering. 5 (3): 2782–2799. doi:10.1016/j.jece.2017.05.029. ISSN 2213-3437.
- Jothirani, R.; Kumar, P. Senthil; Saravanan, A.; Narayan, Abishek S.; Dutta, Abhishek (2016-07-25). "Ultrasonic modified corn pith for the sequestration of dye from aqueous solution". Journal of Industrial and Engineering Chemistry. 39: 162–175. doi:10.1016/j.jiec.2016.05.024. ISSN 1226-086X.
- Gottfried, A.; Shepard, A. D.; Hardiman, K.; Walsh, M. E. (2008-11-01). "Impact of recycling filter backwash water on organic removal in coagulation–sedimentation processes". Water Research. 42 (18): 4683–4691. doi:10.1016/j.watres.2008.08.011. ISSN 0043-1354. PMID 18789473.
- Samal, Sneha (2020-04-15). "Effect of shape and size of filler particle on the aggregation and sedimentation behavior of the polymer composite". Powder Technology. 366: 43–51. doi:10.1016/j.powtec.2020.02.054. ISSN 0032-5910. S2CID 213499533.
- Ahmad, Arslan; Rutten, Sam; de Waal, Luuk; Vollaard, Peter; van Genuchten, Case; Bruning, Harry; Cornelissen, Emile; van der Wal, Albert (2020-06-15). "Mechanisms of arsenate removal and membrane fouling in ferric based coprecipitation–low pressure membrane filtration systems". Separation and Purification Technology. 241: 116644. doi:10.1016/j.seppur.2020.116644. ISSN 1383-5866. S2CID 214445348.
- Nyström, Fredrik; Nordqvist, Kerstin; Herrmann, Inga; Hedström, Annelie; Viklander, Maria (2020-09-01). "Removal of metals and hydrocarbons from stormwater using coagulation and flocculation". Water Research. 182: 115919. doi:10.1016/j.watres.2020.115919. ISSN 0043-1354. PMID 32622122. S2CID 219414366.
- Wang, Lawrence K.; Vaccari, David A.; Li, Yan; Shammas, Nazih K. (2005), "Chemical Precipitation", Physicochemical Treatment Processes, Totowa, NJ: Humana Press, pp. 141–197, doi:10.1385/1-59259-820-x:141, ISBN 978-1-58829-165-3, retrieved 2021-11-12
- Wang, Lawrence K.; Fahey, Edward M.; Wu, Zucheng (2005), Wang, Lawrence K.; Hung, Yung-Tse; Shammas, Nazih K. (eds.), "Dissolved Air Flotation", Physicochemical Treatment Processes, Totowa, NJ: Humana Press, pp. 431–500, doi:10.1385/1-59259-820-x:431, ISBN 978-1-58829-165-3, retrieved 2021-11-12
- Chadha, Utkarsh; Selvaraj, Senthil Kumaran; Vishak Thanu, S.; Cholapadath, Vishnu; Abraham, Ashesh Mathew; Zaiyan, Mohammed; Manikandan, M; Paramasivam, Velmurugan (6 January 2022). "A review of the function of using carbon nanomaterials in membrane filtration for contaminant removal from wastewater". Materials Research Express. doi:10.1088/2053-1591/ac48b8.
- Kurniawan, Tonni Agustiono; Chan, Gilbert Y. S.; Lo, Wai-Hung; Babel, Sandhya (2006-05-01). "Physico–chemical treatment techniques for wastewater laden with heavy metals". Chemical Engineering Journal. 118 (1): 83–98. doi:10.1016/j.cej.2006.01.015. ISSN 1385-8947.
- Vigneswaran, Saravanamuthu; Ngo, Huu Hao; Chaudhary, Durgananda Singh; Hung, Yung-Tse (2005), "Physicochemical Treatment Processes for Water Reuse", Physicochemical Treatment Processes, Totowa, NJ: Humana Press, pp. 635–676, doi:10.1385/1-59259-820-x:635, ISBN 978-1-58829-165-3, retrieved 2021-11-12
- Rengaraj, S; Yeon, Kyeong-Ho; Moon, Seung-Hyeon (October 2001). "Removal of chromium from water and wastewater by ion exchange resins". Journal of Hazardous Materials. 87 (1–3): 273–287. doi:10.1016/s0304-3894(01)00291-6. ISSN 0304-3894. PMID 11566415.
- Singh, N. B.; Nagpal, Garima; Agrawal, Sonal; Rachna (2018-08-01). "Water purification by using Adsorbents: A Review". Environmental Technology & Innovation. 11: 187–240. doi:10.1016/j.eti.2018.05.006. ISSN 2352-1864. S2CID 103693107.
- BABEL, Sandhya; KURNIAWAN, Tonni Agustiono (2003). "A Research Study on Cr(VI) Removal from Contaminated Wastewater Using Natural Zeolite". Journal of Ion Exchange. 14 (Supplement): 289–292. Bibcode:2003JIEx...14S.289B. doi:10.5182/jaie.14.supplement_289. ISSN 1884-3360.
- Sirotkin, A.; Koshkina, L. Yu.; Ippolitov, K. G. (2001). "The BAC-process for treatment of waste water Containing non-ionogenic synthetic surfactants". Water Research. 35 (13): 3265–3271. doi:10.1016/s0043-1354(01)00029-x. PMID 11487125.
- Mezohegyi, Gergo; van der Zee, Frank P.; Font, Josep; Fortuny, Agustí; Fabregat, Azael (2012-07-15). "Towards advanced aqueous dye removal processes: A short review on the versatile role of activated carbon". Journal of Environmental Management. 102: 148–164. doi:10.1016/j.jenvman.2012.02.021. ISSN 0301-4797. PMID 22459012.
- GracePavithra, Kirubanandam; Jaikumar, V.; Kumar, P. Senthil; SundarRajan, PanneerSelvam (2019-08-10). "A review on cleaner strategies for chromium industrial wastewater: Present research and future perspective". Journal of Cleaner Production. 228: 580–593. doi:10.1016/j.jclepro.2019.04.117. ISSN 0959-6526. S2CID 159345994.
- Gray, Nick (2017-01-31). Water Technology (3 ed.). London: CRC Press. doi:10.1201/9781315276106. ISBN 978-1-315-27610-6.
- "Water Desalination". Stanford University. 16 December 2002. Retrieved 29 October 2019.
- Lienhard, John H.; Thiel, Gregory P.; Warsinger, David M.; Banchik, Leonardo D. (2016-12-08). "Low Carbon Desalination: Status and Research, Development, and Demonstration Needs, Report of a workshop conducted at the Massachusetts Institute of Technology in association with the Global Clean Water Desalination Alliance". Prof. Lienhard Via Angie Locknar. Massachusetts Institute of Technology. hdl:1721.1/105755.
- Rouzafay, F.; Shidpour, R. (2020). "Lifetime and dynamics of charge carriers in carbon-incorporated ZnO nanostructures for water treatment under visible light: Femtosecond transient absorption and photoluminescence study". Environmental Chemical Engineering. 8 (5): 104097. doi:10.1016/j.jece.2020.104097. S2CID 219735361.
- "Legislation: The Directive overview". Environment. Brussels: European Commission. 2019-12-31.
- Guidelines for Drinking-water Quality, Fourth Edition; World Health Organization; 2011
- "Environmental quality standards for surface water".
- What is the purpose of drinking water quality guidelines/regulations?. Canada: Safe Drinking Water Foundation. Pdf. Archived 2011-10-06 at the Wayback Machine
- Cicek, V. (2013). "Corrosion and corrosion prevention in boilers". Cathodic protection: industrial solutions for protecting against corrosion. Hoboken, New Jersey: John Wiley & Sons. ISBN 9781118737880.
- Pal, Parimal (2017-01-01), Pal, Parimal (ed.), "Chapter 2 – Chemical Treatment Technology", Industrial Water Treatment Process Technology, Butterworth-Heinemann, pp. 21–63, doi:10.1016/B978-0-12-810391-3.00002-3, ISBN 9780128103913
- Cai, Q.Q.; Lee, B.C.Y.; Ong, S.L.; Hu, J.Y. (February 2021). "Fluidized-bed Fenton technologies for recalcitrant industrial wastewater treatment–Recent advances, challenges and perspective". Water Research. 190: 116692. doi:10.1016/j.watres.2020.116692. PMID 33279748. S2CID 227523802.
- Hansson, Henrik; Kaczala, Fabio; Amaro, Alexandre; Marques, Marcia; Hogland, William (2015-07-01). "Advanced Oxidation Treatment of Recalcitrant Wastewater from a Wood-Based Industry: a Comparative Study of O3 and O3/UV". Water, Air, & Soil Pollution. 226 (7): 229. Bibcode:2015WASP..226..229H. doi:10.1007/s11270-015-2468-5. ISSN 1573-2932. S2CID 92701177.
- Wong, Joe (2013). "Dissolved Air Flotation". Water World. Retrieved 26 June 2020.
- SSWM University. "Slow sand filtration". SSWM University. Retrieved 26 June 2020.
- B. Sizirici Yildiz (2012). "Slow sand filtration". doi:10.1533/9780857096463.3.406.
{{cite journal}}: Cite journal requires|journal=(help) - SSWM University. "Coagulation –Flocculation". SSWM University. Retrieved 26 June 2020.
- "Household Water Treatment Guide". Centre for Affordable Water and Sanitation Technology, Canada. March 2008.
- "Sand as a low-cost support for titanium dioxide photocatalysts". Materials Views. Wiley VCH.
- Lindsten, Don C. (September 1984). "Technology transfer: Water purification, U.S. Army to the civilian community". The Journal of Technology Transfer. 9 (1): 57–59. doi:10.1007/BF02189057. S2CID 154344107.
- "Energy Costs of Water in California". large.stanford.edu. Retrieved 2017-05-07.
- Bhalotra, Sonia R.; Diaz-Cayeros, Alberto; Miller, Grant; Miranda, Alfonso; Venkataramani, Atheendar S. (2021). "Urban Water Disinfection and Mortality Decline in Lower-Income Countries". American Economic Journal: Economic Policy. 13 (4): 490–520. doi:10.1257/pol.20180764. ISSN 1945-7731. S2CID 236955246.
- "Taumata Arowai: a new water regulator". NZ Ministry of Health. 9 June 2022. Retrieved 28 June 2022.
- "Our Drinking Water Quality" (PDF). Simgapore National Water Agency. Retrieved 28 June 2022.
- "Our duties". About us. London: Ofwat (Water Services Regulation Authority). Retrieved 2020-10-23.
- "What We Do". About Us. London: Drinking Water Inspectorate. 2020-06-15.
- "The Water Supply (Water Quality) Regulations 2016". UK Statutory Instruments. London: National Archives, UK. Retrieved 2020-10-23.
- "Water Industry Act 1991". UK Public General Acts. London: National Archives, UK. Retrieved 2020-10-23.
- "Water Quality Regulator says Scotland's tap water quality remains high". News. Edinburgh: Scottish Government. 2019-08-05.
- "Duties of the Drinking Water Inspectorate". Belfast: Northern Ireland Environment Agency. 26 May 2016. Retrieved 2020-10-23.
- "The Water Supply (Water Quality) Regulations (Northern Ireland) 2017". Northern Ireland Statutory Rules. London: National Archives, UK. Retrieved 2020-10-23.
- United States. Safe Drinking Water Act. Pub.L. 93–523; 88 Stat. 1660; 42 U.S.C. § 300f et seq. 1974-12-16.
- "Primacy Enforcement Responsibility for Public Water Systems". Drinking Water Requirements for States and Public Water Systems. Washington, D.C.: United States Environmental Protection Agency (EPA). 2016-11-02.
- Understanding the Safe Drinking Water Act (Report). EPA. June 2004. EPA 816-F-04-030.
- "National Primary Drinking Water Regulations". Ground Water and Drinking Water. EPA. 2019-09-17.
- "Basic Information on the CCL and Regulatory Determination". Contaminant Candidate List. EPA. 2019-07-19.
- "Drinking Water State Revolving Fund". EPA. 2019-10-30.
Further reading
- Eaton, Andrew D.; Franson, Mary Ann H. (2005). Standard methods for the examination of water and wastewater (21 ed.). American Public Health Association. ISBN 978-0-87553-047-5.
External links
- International Water Association Professional / research organization
- NSF International – Independent non-profit standards organization
- WHO.int, WHO Guidelines
- Safe and Sustainable Water for Haiti web site hosted by Grand Valley State University