Anomalocaris
Anomalocaris ("unlike other shrimp", or "abnormal shrimp") is an extinct genus of radiodont, an order of early-diverging stem-group arthropods. The first fossils of Anomalocaris were discovered in the Ogygopsis Shale by Joseph Frederick Whiteaves, with more examples found by Charles Doolittle Walcott in the Burgess Shale.[3] Originally several fossilized parts discovered separately (the mouth, frontal appendages and trunk) were thought to be three separate creatures, a misapprehension corrected by Harry B. Whittington and Derek Briggs in a 1985 journal article.[3][4] With a body length close to 40 centimetres in A. canadensis, Anomalocaris is thought to be one of the earliest examples of an apex predator, though others have been found in older Cambrian lagerstätten deposits.
| Anomalocaris Temporal range: Early Cambrian to Middle Cambrian (Stage 3 to Guzhangian), | |
|---|---|
 | |
| Image of the first complete Anomalocaris fossil found, residing in the Royal Ontario Museum | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Class: | †Dinocaridida |
| Order: | †Radiodonta |
| Family: | †Anomalocarididae |
| Genus: | †Anomalocaris Whiteaves, 1892 |
| Species | |
(10 more unnamed species[2]) | |
Discovery


Anomalocaris has been misidentified several times, in part due to its makeup of a mixture of mineralized and unmineralized body parts; the mouth and frontal appendage was considerably harder and more easily fossilized than the delicate body.[5] Anomalocaris fossils were first collected in 1886[6] or 1888[7] by Richard G. McConnell of the Geological Survey of Canada. The specimens were described and named in 1892 by GSC paleontologist Joseph Frederick Whiteaves.[7] The specimens are now known to represent isolated frontal appendages, but Whiteaves interpreted them as the abdomens of phyllocarid crustaceans. Noting its unusual anatomy for the abdomen of a crustacean, Whiteaves gave it the name Anomalocaris, meaning "unlike other shrimps". In 1928, Danish paleontologist Kai Henriksen proposed that Tuzoia, a Burgess Shale arthropod which was known only from the carapace, represented the missing front half of Anomalocaris.[6] The artists Elie Cheverlange and Charles R. Knight followed this interpretation in their depictions of Anomalocaris.[6]
Unbeknownst to scientists at the time, the body parts of relatives of Anomalocaris had already been described but not recognized as such. The first fossilized mouth of such kind of animal was discovered by Charles Doolittle Walcott, who mistook it for a jellyfish and placed it in the genus Peytoia. Walcott also discovered a frontal appendage but failed to realize the similarities to Whiteaves' discovery and instead identified it as feeding appendage or tail of the coexisted Sidneyia.[5] In the same publication in which he named Peytoia, Walcott named Laggania, a taxon that he interpreted as a holothurian.
In 1966, the Geological Survey of Canada began a comprehensive revision of the Burgess Shale fossil record, led by Cambridge University paleontologist Harry B. Whittington.[6] In the process of this revision, Whittington and his students Simon Conway Morris and Derek Briggs would discover the true nature of Anomalocaris and its relatives, but not without contributing to the history of misinterpretations first.[5] In 1978, Conway Morris recognized that the mouthparts of Laggania were identical to Peytoia, but concluded that Laggania was a composite fossil made up of Peytoia and the sponge Corralio undulata.[8] In 1979, Briggs recognized that the fossils of Anomalocaris were appendages, not abdomens, and proposed that they were the walking legs of a giant arthropod, and that the feeding appendage Walcott had assigned to Sidneyia was the feeding appendage of similar animal, referred to as "appendage F".[9] Later, while clearing what he thought was an unrelated specimen, Harry B. Whittington removed a layer of covering stone to discover the unequivocally connected frontal appendage identical to Anomalocaris and mouthpart similar to Peytoia.[5][3] Whittington linked the two species, but it took several more years for researchers to realize that the continuously juxtaposed Peytoia, Laggania and frontal appendages (Anomalocaris and "appendage F") actually represented a single group of enormous creatures.[4] The two genera have now been placed into the order Radiodonta[6] and are commonly known as radiodonts or anomalocaridids. Since Peytoia was named first, it is the accepted correct name for the entire animal. However, the original frontal appendage was from a larger species distinct from Peytoia and "Laggania" and therefore retains the name Anomalocaris.[10]
In 2011[11] and 2020,[12] compound eyes of Anomalocaris were recovered from a paleontological dig at Emu Bay on Kangaroo Island, Australia, proving that Anomalocaris was indeed an arthropod as had been suspected. The find also indicated that advanced arthropod eyes had evolved very early, before the evolution of jointed legs or hardened exoskeletons.[11]
In 2021, "A." saron[13] and "A." magnabasis[14] were reassigned to the new genus Houcaris, in the family Tamisiocarididae.[15] In the same year, "A." pennsylvanica was reassigned to the genus Lenisicaris.[2] In 2022, specimen ELRC 20001 that was treated as unnamed species of Anomalocaris or whole-body specimen of A. saron got new genus, Innovatiocaris.[16] Multiple phylogenetic analysis also suggest "A". briggsi (tamisiocaridid) and "A". kunmingensis (amplectobeluid) are not species of Anomalocaris as well,[17][18][19][20][21][22][23] and wait to be renamed as the formal species had been done.[12]
Stephen Jay Gould cites Anomalocaris as one of the fossilized extinct species he believed to be evidence of a much more diverse set of phyla that existed in the Cambrian Period,[5] discussed in his book Wonderful Life, a conclusion disputed by other paleontologists.[3]
Anatomy

For the time in which it lived, Anomalocaris was gigantic, up to 38 centimetres (1.25 feet) long excluding the tail fan and frontal appendages.[20] Previous estimation up to 1 metre (3.3 feet)[9] is unlikely based on the ratio of body parts (body length measured only about 2 times the length of frontal appendage in A. canadensis, respectively[20]) and the size of largest frontal appendage (up to 18 centimetres (7.1 inches) in length when extended).[5][20] It propelled itself through the water by undulating the flexible flaps on the sides of its body.[24] Each flap sloped below the one more posterior to it,[25] and this overlapping allowed the lobes on each side of the body to act as a single "fin", maximizing the swimming efficiency.[24] The construction of a remote-controlled model showed this mode of swimming to be intrinsically stable,[26] implying that Anomalocaris would not have needed a complex brain to manage balance while swimming. The body was widest between the third and fifth lobe and narrowed towards the tail, with additional 3 pairs of small flaps on the constricted neck region.[6][27] It is difficult to distinguish lobes near the tail, making an accurate count difficult.[25] For the main trunk flaps, the type species A. canadensis had 13 pairs.[27]


Anomalocaris had an unusual disk-like mouth known as oral cone. The oral cone was composed of several plates organized triradially. Three of the plates were quite large. Three to four medium sized plates could be found between each of the large plates, and several small plates between them. Most of the plates wrinkled and possess scale-like tubercles near the mouth opening.[10][28] Such an oral cone is very different from those of a typical hurdiid radiodont like Peytoia and Hurdia, which is smooth and tetraradial.[10][22] Two large frontal appendage were positioned in front of the mouth, at the front of the head.[6] As a shared character across radiodonts, Anomalocaris also possessed three sclerites on the top and side of its head.[22] The top one, known as a head shield, dorsal carapace or H-element, was shaped like an laterally-elongated[29] oval, with a distinct rim on the outer edge.[27] The remaining two lateral sclerites, known as P-elements, were also ovoid, but connected by a bar-like outgrowth.[22] The P-elements were previously misinterpreted as two huge compound eyes.[27][22]
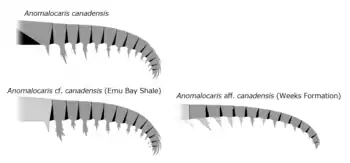
The different species had various bodies. Some had a tail furcae and telson, while others had no tail. And some had a naked body while others were covered by plates.[30]
Anomalocaris usually possessed 14 podomeres (segmental units, at least 1 for shaft and 13 for distal articulated region) on each laterally-flattened[27] frontal appendage, almost each one tipped with a pair of endites (ventral spines).[27] The endites themselves were both equipped with multiple auxiliary spines, which branches off from the anterior and posterior margin of the endites.[13][28][31][27][1] The tail was a large tail fan, composed of three[6][27] pairs of large, lateral fin-shaped lobes and one terminal lobe-like tailpiece.[27] Previous studies suggest the tail fan was used to propel it through Cambrian waters,[3][24] while further hydrodynamic study rather suggest it was more adapted to provide steering function.[32] The gills of the animal, in the form of long, thin, hair-like structures known as lanceolate blades, were arranged in rows forming setal blades. The setal blades were attached by their margin to the top side of the animal, two setal blades per body segment. A divide ran down the middle, separating the gills.[27]
Based on fossilized eyes from the Emu Bay Shale, which belonged to an unnamed Anomalocaris (A. cf. canadensis) from the same stratus[12] (previously thought to be those of "A". briggsi,[11] a species which is unlikely an Anomalocaris[22][15]), the eyes of Anomalocaris were 30 times more powerful than those of trilobites, long thought to have had the most advanced eyes of any contemporary species. With 16,000 lenses, the resolution of the 3-centimetre-wide (1.2 in) eyes would have been rivalled only by that of the modern dragonfly, which has 28,000 lenses in each eye.[11] Additionally, estimation of ecdysozoan opsins suggest that Anomalocaris may have had dichromatic Color vision.[33]
Paleobiology
Diet
.jpg.webp)
The interpretation of Anomalocaris as an active predator is widely accepted throughout the history of research,[4][6][10] as its raptorial frontal appendages and mid-gut glands strongly suggest a predatory lifestyle.[34][35][36] In the case of A. canadensis, its outstanding size amongst Burgess Shale fauna also making it one of the first apex predators known to exist.[36]
However, the long-standing idea that Anomalocaris fed on hard-bodied animals, especially its ability to penetrate mineralized exoskeleton of trilobites, has been questioned.[37][10] Some Cambrian trilobites have been found with round or W-shaped "bite" marks, which were identified as being the same shape as the mouthparts of Peytoia (previously misidentified as those of Anomalocaris[38][10]). Stronger evidence that Anomalocaris ate trilobites comes from coprolite, which contain trilobite parts and are so large that the anomalocarids are the only known organism from that period large enough to have produced them.[38] However, since Anomalocaris lacks any mineralized tissue, it seemed unlikely that it would be able to penetrate the hard, calcified shell of trilobites.[38] Rather, the coprolites may have been produced by a different organisms, such as the trilobites of the genus Redlichia.[28] Another suggested possibility is that Anomalocaris fed by grabbing one end of their prey in its oral cone while using its frontal appendages to quickly rock the other end of the animal back and forth. This produced stresses that exploited the weaknesses of arthropod cuticles, causing the prey's exoskeleton to rupture and allowing the predator to access its innards.[38] This behaviour was originally thought to have provided an evolutionary pressure for trilobites to roll up, to avoid being flexed until they snapped.[38] They could also have attacked soft trilobites that had just molted.[39]
The lack of wear on radiodont mouthparts suggests they did not come into regular contact with mineralized trilobite shells, and were possibly better suited to feeding on smaller, soft-bodied organisms by suction, since they would have experienced structural failure if they were used against the armour of trilobites.[37][28] A. canadensis may have been capable of feeding on organisms with hard exoskeletons due to the short, robust spines on its frontal appendages.[28][14] However, this conclusion is solely based on the comparison with the fragile frontal appendages of suspension feeding radiodonts (e.g. "A". briggsi and Houcaris spp.).[15] As opposed to Peytoia whose oral cone is more rectangular with short protruding spines, the oral cone of A. canadensis has a smaller and more irregular opening, not permitting strong biting motions, and indicating a suction-feeding behavior to suck in softer organisms.[10] Three-dimensional modelling of various radiodont frontal appendages also suggest that A. canadensis is more capable to prey on smaller (2–5 cm in diameter), active, soft-bodied animals (e.g. vetulicolian; free-swimming arthropods; Nectocaris).[35][36]
Paleoecology
Anomalocaris canadensis lived in the Burgess Shale in relatively great numbers, though comparable fossils have been found elsewhere, suggesting a more expansive range over the Laurentian continent.[1] In the Burgess Shale, Anomalocaris is more common in the older sections, notably the Mount Stephen trilobite beds. However, in the younger sections, such as the Phyllopod bed, Anomalocaris could reach much greater sizes; roughly twice the size of its older, trilobite bed relatives. These rare giant specimens have previously been referred to a separate species, Anomalocaris gigantea; however, the validity of this species has been called into question,[9] and is currently synonymized to A. canadensis.[27]
Other unnamed species of Anomalocaris live in vastly different environments.[2] For example, Anomalocaris cf. canadensis (JS-1880) lived in the Maotianshan Shales,[2] a shallow tropical sea or even being delta[40] in what is now modern China. Anomalocaris cf. canadensis (Emu Bay Shale) lived in a comparable environment; the shallow, tropical waters of Cambrian Australia.[2] The Maotianshan Shale and the Emu Bay Shale are very close in proximity, being separated by a small landmass, far from the Burgess Shale.[2] These two locations also included "Anomalocaris" kunmingensis and "Anomalocaris" briggsi respectively, species that previously attributed[41][42][28][43] but taxonomically unlikely to be a member of Anomalocaris nor even Anomalocarididae.[2][44]
See also
- 8564 Anomalocaris, an asteroid named after this animal
- Radiodonta, extinct arthropod order compose of Anomalocaris and its relatives
- Houcaris, Lenisicaris and Innovatiocaris, radiodont genera contain species originally named as Anomalocaris
- Aegirocassis, a giant filter-feeding radiodont from Ordovician Morocco
- Cambrian explosion
- Opabinia
- Wiwaxia
Footnotes
- Lerosey-Aubril R, Hegna TA, Babcock LE, Bonino E, Kier C (2014-05-19). "Arthropod appendages from the Weeks Formation Konservat-Lagerstätte: new occurrences of anomalocaridids in the Cambrian of Utah, USA". Bulletin of Geosciences: 269–282. doi:10.3140/bull.geosci.1442.
- Wu Y, Ma J, Lin W, Sun A, Zhang X, Fu D (2021). "New anomalocaridids (Panarthropoda: Radiodonta) from the lower Cambrian Chengjiang Lagerstätte: Biostratigraphic and paleobiogeographic implications". Palaeogeography, Palaeoclimatology, Palaeoecology. 569: Article 110333. Bibcode:2021PPP...569k0333W. doi:10.1016/j.palaeo.2021.110333. S2CID 233565727.
- Conway Morris S (1998). The crucible of creation: the Burgess Shale and the rise of animals. Oxford [Oxfordshire]: Oxford University Press. pp. 56–9. ISBN 978-0-19-850256-2.
- Whittington HB, Briggs DE (1985). "The largest Cambrian animal, Anomalocaris, Burgess Shale, British Columbia". Philosophical Transactions of the Royal Society B. 309 (1141): 569–609. Bibcode:1985RSPTB.309..569W. doi:10.1098/rstb.1985.0096.
- Gould SJ (1989). Wonderful life: the Burgess Shale and the nature of history. New York: W.W. Norton. pp. 194–206. ISBN 978-0-393-02705-1.
- Collins D (1996). "The "Evolution" of Anomalocaris and Its Classification in the Arthropod Class Dinocarida (nov.) and Order Radiodonta (nov.)". Journal of Paleontology. 70 (2): 280–293. doi:10.1017/S0022336000023362. JSTOR 1306391. S2CID 131622496.
- Whiteaves JF (1892). "Description of a new genus and species of phyllocarid Crustacea from the Middle Cambrian of Mount Stephen, B. C.". The Canadian Record of Science. 5 (4).
- Conway Morris S (1978). "Laggania cambria Walcott: A Composite Fossil". Journal of Paleontology. 52 (1): 126–131. JSTOR 1303799.
- Briggs DE (1979). "Anomalocaris, the largest known Cambrian arthropod". Palaeontology. 22 (3): 631–664.
- Daley AC, Bergström J (June 2012). "The oral cone of Anomalocaris is not a classic peytoia". Die Naturwissenschaften. 99 (6): 501–4. Bibcode:2012NW.....99..501D. doi:10.1007/s00114-012-0910-8. PMID 22476406. S2CID 2042726.
- Paterson JR, García-Bellido DC, Lee MS, Brock GA, Jago JB, Edgecombe GD (December 2011). "Acute vision in the giant Cambrian predator Anomalocaris and the origin of compound eyes". Nature. 480 (7376): 237–40. Bibcode:2011Natur.480..237P. doi:10.1038/nature10689. PMID 22158247. S2CID 2568029.
- Paterson JR, Edgecombe GD, García-Bellido DC (December 2020). "Disparate compound eyes of Cambrian radiodonts reveal their developmental growth mode and diverse visual ecology". Science Advances. 6 (49): eabc6721. Bibcode:2020SciA....6.6721P. doi:10.1126/sciadv.abc6721. hdl:10141/622906. PMC 7821881. PMID 33268353.
- Xian-Guang H, Bergström J, Ahlberg P (1995-09-01). "Anomalocaris and other large animals in the lower Cambrian Chengjiang fauna of southwest China". GFF. 117 (3): 163–183. doi:10.1080/11035899509546213. ISSN 1103-5897.
- Pates S, Daley AC, Edgecombe GD, Cong P, Lieberman BS (2019). "Systematics, preservation and biogeography of radiodonts from the southern Great Basin, USA, during the upper Dyeran (Cambrian Series 2, Stage 4)". Papers in Palaeontology. 7: 235–262. doi:10.1002/spp2.1277. ISSN 2056-2799. S2CID 204260554.
- Wu Y, Fu D, Ma J, Lin W, Sun A, Zhang X (2021). "Houcaris gen. nov. from the early Cambrian (Stage 3) Chengjiang Lagerstätte expanded the palaeogeographical distribution of tamisiocaridids (Panarthropoda: Radiodonta)". PalZ. 95 (2): 209–221. doi:10.1007/s12542-020-00545-4. ISSN 1867-6812. S2CID 235221043.
- Zeng, Han; Zhao, Fangchen; Zhu, Maoyan (2022-09-07). "Innovatiocaris, a complete radiodont from the early Cambrian Chengjiang Lagerstätte and its implications for the phylogeny of Radiodonta". Journal of the Geological Society. doi:10.1144/jgs2021-164. ISSN 0016-7649. S2CID 252147346.
- Vinther J, Stein M, Longrich NR, Harper DA (March 2014). "A suspension-feeding anomalocarid from the Early Cambrian" (PDF). Nature. 507 (7493): 496–9. Bibcode:2014Natur.507..496V. doi:10.1038/nature13010. PMID 24670770. S2CID 205237459.
- Cong P, Ma X, Hou X, Edgecombe GD, Strausfeld NJ (September 2014). "Brain structure resolves the segmental affinity of anomalocaridid appendages". Nature. 513 (7519): 538–42. Bibcode:2014Natur.513..538C. doi:10.1038/nature13486. PMID 25043032. S2CID 4451239.
- Van Roy P, Daley AC, Briggs DE (June 2015). "Anomalocaridid trunk limb homology revealed by a giant filter-feeder with paired flaps". Nature. 522 (7554): 77–80. Bibcode:2015Natur.522...77V. doi:10.1038/nature14256. PMID 25762145. S2CID 205242881.
- Lerosey-Aubril R, Pates S (September 2018). "New suspension-feeding radiodont suggests evolution of microplanktivory in Cambrian macronekton". Nature Communications. 9 (1): 3774. Bibcode:2018NatCo...9.3774L. doi:10.1038/s41467-018-06229-7. PMC 6138677. PMID 30218075.
- Liu J, Lerosey-Aubril R, Steiner M, Dunlop JA, Shu D, Paterson JR (2018-11-01). "Origin of raptorial feeding in juvenile euarthropods revealed by a Cambrian radiodontan". National Science Review. 5 (6): 863–869. doi:10.1093/nsr/nwy057. ISSN 2095-5138.
- Moysiuk J, Caron JB (August 2019). "A new hurdiid radiodont from the Burgess Shale evinces the exploitation of Cambrian infaunal food sources". Proceedings. Biological Sciences. 286 (1908): 20191079. doi:10.1098/rspb.2019.1079. PMC 6710600. PMID 31362637.
- Moysiuk J, Caron JB (2021). "Exceptional multifunctionality in the feeding apparatus of a mid-Cambrian radiodont". Paleobiology. 47 (4): 704–724. doi:10.1017/pab.2021.19. ISSN 0094-8373. S2CID 236552819.
- Usami Y (January 2006). "Theoretical study on the body form and swimming pattern of Anomalocaris based on hydrodynamic simulation". Journal of Theoretical Biology. 238 (1): 11–7. Bibcode:2006JThBi.238...11U. doi:10.1016/j.jtbi.2005.05.008. PMID 16002096.
- Whittington HB, Briggs DE (1985). "The Largest Cambrian Animal, Anomalocaris, Burgess Shale, British Columbia". Philosophical Transactions of the Royal Society B (free full text). 309 (1141): 569–609. Bibcode:1985RSPTB.309..569W. doi:10.1098/rstb.1985.0096.
- Briggs DE (May 1994). "Giant predators from the cambrian of china". Science. 264 (5163): 1283–4. Bibcode:1994Sci...264.1283B. doi:10.1126/science.264.5163.1283. PMID 17780843.
- Daley AC, Edgecombe GD (January 2014). "Morphology of Anomalocaris canadensis from the Burgess Shale". Journal of Paleontology. 88 (1): 68–91. doi:10.1666/13-067. S2CID 86683798.
- Daley AC, Paterson JR, Edgecombe GD, García-Bellido DC, Jago JB (2013). Donoghue P (ed.). "New anatomical information on Anomalocaris from the Cambrian Emu Bay Shale of South Australia and a reassessment of its inferred predatory habits". Palaeontology: n/a. doi:10.1111/pala.12029.
- Zeng H, Zhao F, Yin Z, Zhu M (2018-01-02). "Morphology of diverse radiodontan head sclerites from the early Cambrian Chengjiang Lagerstätte, south-west China". Journal of Systematic Palaeontology. 16 (1): 1–37. doi:10.1080/14772019.2016.1263685. ISSN 1477-2019. S2CID 133549817.
- Background on Anomalocaris part 3 - Trilobites.info
- Liu Q (2013-09-01). "The first discovery of anomalocaridid appendages from the Balang Formation (Cambrian Series 2) in Hunan, China". Alcheringa: An Australasian Journal of Palaeontology. 37 (3): 338–343. doi:10.1080/03115518.2013.753767. ISSN 0311-5518. S2CID 129212098.
- Sheppard KA, Rival DE, Caron JB (October 2018). "On the Hydrodynamics of Anomalocaris Tail Fins". Integrative and Comparative Biology. 58 (4): 703–711. doi:10.1093/icb/icy014. hdl:1974/22737. PMID 29697774.
- Fleming JF, Kristensen RM, Sørensen MV, Park TS, Arakawa K, Blaxter M, et al. (December 2018). "Molecular palaeontology illuminates the evolution of ecdysozoan vision". Proceedings. Biological Sciences. 285 (1892): 20182180. doi:10.1098/rspb.2018.2180. PMC 6283943. PMID 30518575.
- Vannier J, Liu J, Lerosey-Aubril R, Vinther J, Daley AC (May 2014). "Sophisticated digestive systems in early arthropods". Nature Communications. 5 (1): 3641. Bibcode:2014NatCo...5.3641V. doi:10.1038/ncomms4641. PMID 24785191.
- De Vivo G, Lautenschlager S, Vinther J (16 December 2016). Reconstructing anomalocaridid feeding appendage dexterity sheds light on radiodontan ecology (Report).
- De Vivo G, Lautenschlager S, Vinther J (July 2021). "Three-dimensional modelling, disparity and ecology of the first Cambrian apex predators". Proceedings. Biological Sciences. 288 (1955): 20211176. doi:10.1098/rspb.2021.1176. PMC 8292756. PMID 34284622.
- Hagadorn JW (August 2009). "Taking a Bite out of Anomalocaris" (PDF). In Smith MR, O'Brien LJ, Caron J (eds.). Abstract Volume. International Conference on the Cambrian Explosion (Walcott 2009). Toronto, Ontario, Canada: The Burgess Shale Consortium (published 31 July 2009). ISBN 978-0-9812885-1-2.
- Nedin C (1999). "Anomalocaris predation on nonmineralized and mineralized trilobites". Geology. 27 (11): 987–990. Bibcode:1999Geo....27..987N. doi:10.1130/0091-7613(1999)027<0987:APONAM>2.3.CO;2.
- Ancient shrimp monster not so fierce after all - Phys.org
- Saleh, Farid; Qi, Changshi; Buatois, Luis A.; Mángano, M. Gabriela; Paz, Maximiliano; Vaucher, Romain; Zheng, Quanfeng; Hou, Xian-Guang; Gabbott, Sarah E.; Ma, Xiaoya (2022-03-23). "The Chengjiang Biota inhabited a deltaic environment". Nature Communications. 13 (1): 1569. Bibcode:2022NatCo..13.1569S. doi:10.1038/s41467-022-29246-z. ISSN 2041-1723. PMC 8943010. PMID 35322027.
- Nedin, Christopher (1995). The Emu Bay Shale, a Lower Cambrian fossil Lagerstatte, Kangaroo Island, South Australia.
- Wang Y, Huang D, Hu S (2013-11-01). "New anomalocardid frontal appendages from the Guanshan biota, eastern Yunnan". Chinese Science Bulletin. 58 (32): 3937–3942. Bibcode:2013ChSBu..58.3937W. doi:10.1007/s11434-013-5908-x. ISSN 1861-9541.
- Jeanes J. "Mapping the world's Burgess Shale-type deposits". www.virtualmuseum.ca. Retrieved 2019-09-16.
- Jiao DG, Pates S, Lerosey-Aubril R, Ortega-Hernández J, Yang J, Lan T, Zhang XG (2021). "The endemic radiodonts of the Cambrian Stage 4 Guanshan biota of South China". Acta Palaeontologica Polonica. 66. doi:10.4202/app.00870.2020. ISSN 0567-7920.
External links
- "Anomalocaris canadensis". Burgess Shale Fossil Gallery. Virtual Museum of Canada. 2011.
- Anomalocaris 'homepage' with swimming animation
- Burgess Shale: Anomalocaris canadensis (proto-arthropod), Smithsonian.
