Diatomaceous earth
Diatomaceous earth (/ˌdaɪ.ətəˈmeɪʃəs/), diatomite (/daɪˈætəˌmaɪt/), or kieselgur/kieselguhr is a naturally occurring, soft, siliceous sedimentary rock that can be crumbled into a fine white to off-white powder. It has a particle size ranging from more than 3 μm to less than 1 mm, but typically 10 to 200 μm. Depending on the granularity, this powder can have an abrasive feel, similar to pumice powder, and has a low density as a result of its high porosity. The typical chemical composition of oven-dried diatomaceous earth is 80–90% silica, with 2–4% alumina (attributed mostly to clay minerals), and 0.5–2% iron oxide.[1]

Diatomaceous earth consists of the fossilized remains of diatoms, a type of hard-shelled microalgae. It is used as a filtration aid, mild abrasive in products including metal polishes and toothpaste, mechanical insecticide, absorbent for liquids, matting agent for coatings, reinforcing filler in plastics and rubber, anti-block in plastic films, porous support for chemical catalysts, litter boxes, activator in coagulation studies, a stabilizing component of dynamite, a thermal insulator, and a soil for potted plants and trees as in the art of bonsai.[2][3]
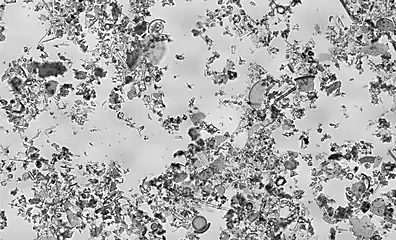

Composition
Each deposit of diatomaceous earth is different, with varying blends of pure diatomaceous earth combined with other natural clays and minerals. The diatoms in each deposit contain different amounts of silica, depending on the sedimentation conditions, on the presence of other sediments (clay, sand, volcanic ashes), and on the age of the deposit (diagenesis, silica (SiO2) dissolution/precipitation, diatoms tests ageing). The species of diatom may also differ among deposits. The species of diatom is dependent upon the age and paleoecology of the deposit. In turn, the shape of a diatom is determined by its species.
Many deposits throughout British Columbia, such as Red Lake Earth, are from the Miocene epoch and contain a species of diatom known as Melosira granulata. These diatoms are approximately 12 to 13 million years old and have a small globular shape. A deposit containing diatoms from this epoch can provide certain benefits over others. For example, diatoms from the Eocene epoch (approximately 40 to 50 million years old) are not as effective in their ability to absorb fluids because as older diatoms recrystallize, their small pores become filled with silica.[4]
Formation
| Part of a series related to |
| Biomineralization |
|---|
 |
|
Diatomite forms by the accumulation of the amorphous silica (opal, SiO2·nH2O) remains of dead diatoms (microscopic single-celled algae) in lake sediment or marine sediments. The fossil remains consist of a pair of symmetrical shells or frustules.[1] Marine diatomites are found in association with a wide variety of other rock types but lacustrine diatomites are almost always associated with volcanic rock. Diatomaceous chert consists of diatomite that has been cemented with silica.[5]
Diatoms are able to extract silica from water that is less than 1% saturated in amorphous silica (saturation index (SI): -2). Their frustules remain undissolved because they are surrounded by an organic matrix. Clay minerals may also precipitate on the frustules and protect them from dissolution in sea water. When the diatom dies, the frustule is stripped of its organic layer and exposed to sea water. As a result, only 1% to 10% of frustules survive long enough to be buried under sediments and some of this is dissolved within the sediments. Only an estimated 0.05% to 0.15% of the original amount of silica produced by diatoms is preserved in the sedimentary record.[6]
Discovery
In 1836 or 1837, German peasant Peter Kasten discovered diatomaceous earth (German: Kieselgur) when sinking a well on the northern slopes of the Haußelberg hill, in the Lüneburg Heath in North Germany.[7][8]
The extraction site on the Lüneburg Heath was 1863–1994 Neuohe, while the storage sites were:
| Storage site | from | to |
|---|---|---|
| Wiechel | 1871 | 1978 |
| Hützel | 1876 | 1969 |
| Hösseringen | c. 1880 | 1894 |
| Hammerstorf | c. 1880 | 1920 |
| Oberohe | 1884 | 1970 |
| Schmarbeck | 1896 | c. 1925 |
| Steinbeck | 1897 | 1928 |
| Breloh | 1907 | 1975 |
| Schwindebeck | 1913 | 1973 |
| Hetendorf | 1970 | 1994 |
The deposits are up to 28 meters (92 ft) thick and are all of freshwater diatomaceous earth.
 c. 1900–1910 Diatomaceous earth pit at Neuohe
c. 1900–1910 Diatomaceous earth pit at Neuohe c. 1900–1910 a drying area: one firing pile is being prepared; another is under way
c. 1900–1910 a drying area: one firing pile is being prepared; another is under way 1913: Staff at the Neuohe factory, with male workers and a female cook in front of a drying shed
1913: Staff at the Neuohe factory, with male workers and a female cook in front of a drying shed
Until World War I, almost the entire worldwide production of diatomaceous earth was from this region.
Other deposits
In Poland diatomaceous earth deposits are found in Jawornik, and are composed mostly of diatomaceous skeletons (frustules) [9]
In Germany, diatomaceous earth was also extracted at Altenschlirf[10] on the Vogelsberg (Upper Hesse) and at Klieken[11] (Saxony-Anhalt).
There is a layer of diatomaceous earth more than 6 meters (20 ft) thick in the nature reserve of Soos in the Czech Republic.[12]
Deposits on the Isle of Skye, off the west coast of Scotland, were mined until 1960.[13]
In Colorado and in Clark County, Nevada, United States, there are deposits that are up to several hundred meters thick in places. Marine deposits have been worked in the Sisquoc Formation in Santa Barbara County, California near Lompoc and along the Southern California coast. This is the world's largest deposit of diatomite.[14] Additional marine deposits have been worked in Maryland, Virginia, Algeria and the MoClay of Denmark. Freshwater lake deposits occur in Nevada, Oregon, Washington and California. Lake deposits also occur in interglacial lakes in the eastern United States, in Canada and in Europe in Germany, France, Denmark and the Czech Republic. The worldwide association of diatomite deposits and volcanic deposits suggests that the availability of silica from volcanic ash may be necessary for thick diatomite deposits.[15]
Diatomaceous earth is sometimes found on desert surfaces. Research has shown that the erosion of diatomaceous earth in such areas (such as the Bodélé Depression in the Sahara) is one of the most important sources of climate-affecting dust in the atmosphere.[16]
The siliceous frustules of diatoms accumulate in fresh and brackish wetlands and lakes. Some peats and mucks contain a sufficient abundance of frustules such that they can be mined. Most of Florida's diatomaceous earths have been found in the muck of wetlands or lakes. The American Diatomite Corporation, from 1935 to 1946, refined a maximum of 145 tons per year from their processing plant near Clermont, Florida. Muck from several locations in Lake County, Florida was dried and burned (calcined) to produce the diatomaceous earth.[17] It was formerly extracted from Lake Mývatn in Iceland.
The commercial deposits of diatomite are restricted to Tertiary or Quaternary periods. Older deposits from as early as the Cretaceous Period are known, but are of low quality.[15]
Diatomite deposits rich in fossils have been located in New Zealand, but mining of the Foulden Maar deposits on an industrial scale, for conversion to animal feed, has drawn strong opposition.[18]
Commercial form
Diatomaceous earth is available commercially in several formats:
- granulated diatomaceous earth is a raw material simply crushed for convenient packaging
- milled or micronized diatomaceous earth is especially fine (10 μm to 50 μm) and used for insecticides.
- calcined diatomaceous earth is heat-treated and activated for filters.
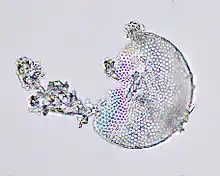

Usages
Explosives
In 1866, Alfred Nobel discovered that nitroglycerin could be made much more stable if absorbed in diatomite (kieselguhr). This allowed a much safer transport and handling than pure nitroglycerin under the liquid form. Nobel patented this mixture as dynamite in 1867; the mixture is also called guhr dynamite by reference to the German term kieselguhr.[19]
Filtration
The Celle engineer, Wilhelm Berkefeld, recognized the ability of the diatomaceous earth to filter and developed tubular filters (known as filter candles) fired from diatomaceous earth.[20] During the cholera epidemic in Hamburg in 1892, these Berkefeld filters were used successfully. One form of diatomaceous earth is used as a filter medium, especially for swimming pools. It has a high porosity because it is composed of microscopically small, hollow particles. Diatomaceous earth (sometimes referred to by trademarked brand names such as Celite) is used in chemistry as a filtration aid, to increase flow rate, and filter very fine particles that would otherwise pass through or clog filter paper. It is also used to filter water, particularly in the drinking water treatment process and in fish tanks, and other liquids, such as beer and wine. It can also filter syrups, sugar, and honey without removing or altering their color, taste, or nutritional properties.[21]
Abrasive
The oldest use of diatomite is as a very mild abrasive and has been used in toothpaste, metal polishes, and some facial scrubs.
Pest control
Diatomite is of value as an insecticide because of its abrasive and physico-sorptive properties.[22] The fine powder adsorbs lipids from the waxy outer layer of the exoskeletons of many species of insects; this layer acts as a barrier that resists the loss of water vapour from the insect's body. Damaging the layer increases the evaporation of water from their bodies, so that they dehydrate, often fatally.
Arthropods die as a result of the water pressure deficiency, based on Fick's laws of diffusion. This also works against gastropods and is commonly employed in gardening to defeat slugs. However, since slugs inhabit humid environments, efficacy is very low. Diatomaceous earth is sometimes mixed with an attractant or other additives to increase its effectiveness.
The shape of the diatoms contained in a deposit has not been proven to affect their functionality when it comes to the adsorption of lipids; however, certain applications, such as that for slugs and snails, do work best when a particularly shaped diatom is used, suggesting that lipid adsorption is not the whole story. For example, in the case of slugs and snails, large, spiny diatoms work best to lacerate the epithelium of the mollusk. Diatom shells will work to some degree on the vast majority of animals that undergo ecdysis in shedding cuticle, such as arthropods or nematodes. It also may have other effects on lophotrochozoans, such as mollusks or annelids.
Medical-grade diatomite has been studied for its efficacy as a deworming agent in cattle; in both studies cited the groups being treated with diatomaceous earth did not fare any better than control groups.[23][24] It is commonly used in lieu of boric acid and can be used to help control and possibly eliminate bed bugs,[25] house dust mite, cockroach, ant, and flea infestations.[26]
Diatomaceous earth is widely applied for insect control in grain storage.[27]
In order to be effective as an insecticide, diatomaceous earth must be uncalcinated (i.e., it must not be heat-treated prior to application)[28] and have a mean particle size below about 12 μm (i.e., food grade—see below).
Although considered to be relatively low-risk, pesticides containing diatomaceous earth are not exempt from regulation in the United States under the Federal Insecticide, Fungicide, and Rodenticide Act and must be registered with the Environmental Protection Agency.[29]
Thermal
Its thermal properties enable it to be used as the barrier material in some fire-resistant safes. It is also used in evacuated powder insulation for use with cryogenics.[30] Diatomaceous earth powder is inserted into the vacuum space to aid in the effectiveness of vacuum insulation. It was used in the classical AGA cookers as a thermal heat barrier.
Catalyst support
Diatomaceous earth also finds some use as a support for catalysts, generally serving to maximize a catalyst's surface area and activity. For example, nickel can be supported on the material—the combination is called Ni–Kieselguhr—to improve its activity as a hydrogenation catalyst.[31]
Agriculture
Natural freshwater diatomaceous earth is used in agriculture for grain storage as an anticaking agent, as well as an insecticide.[32] It is approved by the Food and Drug Administration as a feed additive[33] to prevent caking.[34]
Some believe it may be used as a natural anthelmintic (dewormer), although studies have not shown it to be effective.[23][24] Some farmers add it to their livestock and poultry feed to prevent the caking of feed.[35] "Food-Grade Diatomaceous Earth" is widely available in agricultural feed supply stores.
Freshwater diatomite can be used as a growing medium in hydroponic gardens.
It is also used as a growing medium in potted plants, particularly as bonsai soil. Bonsai enthusiasts use it as a soil additive, or pot a bonsai tree in 100% diatomaceous earth. In vegetable gardening it is sometimes used as a soil conditioner, because like perlite, vermiculite, and expanded clay, it retains water and nutrients, while draining fast and freely, allowing high oxygen circulation within the growing medium.
Marker in livestock nutrition experiments
Natural dried, not calcinated diatomaceous earth is regularly used in livestock nutrition research as a source of acid-insoluble ash (AIA), which is used as an indigestible marker. By measuring the content of AIA relative to nutrients in test diets and feces or digesta sampled from the terminal ileum (last third of the small intestine) the percentage of that nutrient digested can be calculated using the following equation:
where:
Natural freshwater diatomaceous earth is preferred by many researchers over chromic oxide, which has been widely used for the same purpose, the latter being a known carcinogen and, therefore, a potential hazard to research personnel.
Construction
Spent diatomaceous earth from the brewing process can be added to ceramic mass for the production of red bricks with higher open porosity.[36]
Diatomaceous earth is considered a very prominent inorganic non-metallic material that can be utilized for the production of various ceramics, including production of porous ceramics under low temperature hydrothermal technology.[37]
Specific varieties
- Tripolite is the variety found in Tripoli, Libya.
- Bann clay is the variety found in the Lower Bann valley in Northern Ireland.
- Moler (mo-clay) is the variety found in northwestern Denmark, especially on the islands of Fur and Mors.
- Freshwater-derived food grade diatomaceous earth is the type used in United States agriculture for grain storage, as feed supplement, and as an insecticide. It is produced uncalcinated, has a very fine particle size, and is very low in crystal silica (<2%).
- Salt-water-derived pool / beer / wine filter grade is not suitable for human consumption or effective as an insecticide. Usually calcinated before being sold to remove impurities and undesirable volatile contents, it is composed of larger particles than the freshwater version and has a high crystalline silica content (>60%).
Microbial degradation
Certain species of bacteria in oceans and lakes can accelerate the rate of dissolution of silica in dead and living diatoms by using hydrolytic enzymes to break down the organic algal material.[38][39]
Climatologic importance
The Earth's climate is affected by dust in the atmosphere, so locating major sources of atmospheric dust is important for climatology. Recent research indicates that surface deposits of diatomaceous earth play an important role. Research shows that significant dust comes from the Bodélé Depression in Chad, where storms push diatomite gravel over dunes, generating dust by abrasion.[40]
Safety considerations
Inhalation of crystalline silica is harmful to the lungs, causing silicosis. Amorphous silica is considered to have low toxicity, but prolonged inhalation causes changes to the lungs.[41] Diatomaceous earth is mostly amorphous silica but contains some crystalline silica, especially in the saltwater forms.[42] In a 1978 study of workers, those exposed to natural diatomaceous earth for over five years had no significant lung changes while 40% of those exposed to the calcined form had developed pneumoconiosis.[43] Today's common diatomaceous earth formulations are safer to use, as they are predominantly made up of amorphous silica and contain little or no crystalline silica.[44]
The crystalline silica content of diatomaceous earth is regulated in the United States by the Occupational Safety and Health Administration (OSHA) and there are guidelines from the National Institute for Occupational Safety and Health that set maximum amounts allowable in the product (1%) and in the air near the breathing zone of workers, with a recommended exposure limit at 6 mg/m3 over an 8-hour workday.[44] OSHA has set a permissible exposure limit for diatomaceous earth as 20 mppcf (80 mg/m3/%SiO2). At levels of 3,000 mg/m3, diatomaceous earth is immediately dangerous to life and health.[45]
In the 1930s, long-term occupational exposure among workers in the cristobalite diatomaceous earth industry who were exposed to high levels of airborne crystalline silica over decades were found to have an increased risk of silicosis.[46]
Today, workers are required to use respiratory-protection measures when concentrations of silica exceed allowable levels.
Diatomite produced for pool filters is treated with high heat (calcination) and a fluxing agent (soda ash), causing the formerly harmless amorphous silicon dioxide to assume its crystalline form.[44]
See also
- Aerogel – Synthetic ultralight solid material
- Biomineralization – Process by which living organisms produce minerals
- Fuller's earth – Any clay material that can decolorise oil or other liquids
- Perlite – Amorphous volcanic glass
- Rock flour – Glacier-generated sediment
- Siliceous ooze – Biogenic pelagic sediment located on the deep ocean floor
- Zeolite – Microporous, aluminosilicate mineral
References
- Antonides, Lloyd E. (1997). Diatomite (PDF). USGS. Retrieved December 12, 2010.
- Reka, Arianit A.; Pavlovski, Blagoj; Ademi, Egzon; Jashari, Ahmed; Boev, Blazo; Boev, Ivan; Makreski, Petre (December 31, 2019). "Effect Of Thermal Treatment Of Trepel At Temperature Range 800-1200˚C". Open Chemistry. 17 (1): 1235–1243. doi:10.1515/chem-2019-0132.
- Reka, Arianit; Anovski, Todor; Bogoevski, Slobodan; Pavlovski, Blagoj; Boškovski, Boško (December 29, 2014). "Physical-chemical and mineralogical-petrographic examinations of diatomite from deposit near village of Rožden, Republic of Macedonia". Geologica Macedonica. 28 (2): 121–126.
- "Diatoms". UCL London's Global University. Retrieved September 14, 2011.
- Boggs, Sam (2006). Principles of sedimentology and stratigraphy (4th ed.). Upper Saddle River, N.J.: Pearson Prentice Hall. p. 208. ISBN 0131547283.
- Blatt, Harvey; Middleton, Gerard; Murray, Raymond (1980). Origin of sedimentary rocks (2d ed.). Englewood Cliffs, N.J.: Prentice-Hall. pp. 578–579. ISBN 0136427103.
- Ghobara, Mohamed M; Mazumder, Nirmal; Vinayak, Vandana; Reissig, Louisa; Gebeshuber, Ille C; Tiffany, Mary Ann; Gordon, Richard; Gordon, Richard (June 28, 2019). "On Light and Diatoms: A Photonics and Photobiology Review". Diatoms: Fundamentals and Applications: 475. doi:10.1002/9781119370741.ch7. ISBN 9781119370215. S2CID 202096365.
- Klebs, Florian (December 17, 2001). "Deutschland - Wiege des Nobelpreis: Tourismus-Industrie und Forschung auf den Spuren Alfred Nobels" (in German). Alexander von Humboldt Foundation. Archived from the original on November 17, 2002. Retrieved October 12, 2018.
- Lutyński, Marcin; Sakiewicz, Piotr; Lutyńska, Sylwia (October 31, 2019). "Characterization of Diatomaceous Earth and Halloysite Resources of Poland". Minerals. 9 (11): 670. Bibcode:2019Mine....9..670L. doi:10.3390/min9110670. ISSN 2075-163X.
- "Was ist es um die Kieselgur?". Archived from the original on September 28, 2007. Retrieved March 10, 2010. Über den früheren Abbau von Kieselgur im Vogelsberg/Hessen
- Geschichte des Kieselgurabbaus in Klieken Archived April 20, 2008, at the Wayback Machine
- "Protokol o vypořádání připomínek a schválení plánu péče NPR Soos na období 2016–2023" [Protocol on the settlement of comments and approval of the NPR Soos care plan for the period 2016-2023] (in Czech). Ministerstvo životního prostředí [Ministry of the Environment]. February 1, 2016. p. 13. Retrieved April 5, 2021.
- "Skye diatomite: A lost industry". www.stornowaygazette.co.uk.
- Rice, Stanley (July–August 2020). "Creationist Funhouse, Episode Four: God Plays In The Mud". Skeptical Inquirer. Amherst, New York: Center for Inquiry. Archived from the original on March 4, 2021. Retrieved March 4, 2021.
- Cummins, Arthur B., Diatomite, in Industrial Minerals and Rocks, 3rd ed. 1960, American Institute of Mining, Metallurgical, and Petroleum Engineers, pp. 303–319
- Todd, Martin C.; Washington, Richard; Martins, José Vanderlei; Dubovik, Oleg; Lizcano, Gil; M'Bainayel, Samuel; Engelstaedter, Sebastian (March 22, 2007). "Mineral dust emission from the Bodélé Depression, northern Chad, during BoDEx 2005". Journal of Geophysical Research. 112 (D6): D06207. Bibcode:2007JGRD..112.6207T. doi:10.1029/2006JD007170.
- Davis, John H. Jr. (1946). The Peat Deposits of Florida Their Occurrence, Development and Uses, Geological Bulletin No. 30. Florida Geological Survey.
- Hancock, Farah (May 13, 2019). "Opposition grows to fossil mining". Newsroom.co.nz. Retrieved January 21, 2021.
{{cite news}}: CS1 maint: url-status (link) - Rustan, Agne (February 1, 1998). Rock Blasting Terms and Symbols: A Dictionary of Symbols and Terms in Rock Blasting and Related Areas like Drilling, Mining and Rock Mechanics. Taylor & Francis. p. 83. ISBN 978-1-4665-7178-5.
Bulson, P.S. (July 24, 1997). Explosive Loading of Engineering Structures. CRC Press. p. 3. ISBN 978-1-135-82980-3. - "Berkefeld & Aquantis Water Treatment – Veolia Water Technologies". technomaps.veoliawatertechnologies.com.
- Amos Ives Root; Ernest Rob Root (March 1, 2005). The ABC And Xyz of Bee Culture. Kessinger Publishing. p. 387. ISBN 978-1-4179-2427-1.
- Fields, Paul; Allen, Sylvia; Korunic, Zlatko; McLaughlin, Alan; Stathers, Tanya (July 2002). "Standardized testing for diatomaceous earth" (PDF). Proceedings of the Eighth International Working Conference of Stored-Product Protection. York, U.K.: Entomological Society of Manitoba.
- Lartigue, E. del C.; Rossanigo, C. E. (2004). "Insecticide and anthelmintic assessment of diatomaceous earth in cattle". Veterinaria Argentina. 21 (209): 660–674.
- Fernandez, M. I.; Woodward, B. W.; Stromberg, B. E. (1998). "Effect of diatomaceous earth as an anthelmintic treatment on internal parasites and feedlot performance of beef steers". Animal Science. 66 (3): 635–641. doi:10.1017/S1357729800009206.
- "Bed Bug Control With Diatomaceous Earth". Absorbent Products. October 29, 2020. Retrieved October 29, 2020.
- Faulde, M. K.; Tisch, M.; Scharninghausen, J. J. (August 2006). "Efficacy of modified diatomaceous earth on different cockroach species (Orthoptera, Blattellidae) and silverfish (Thysanura, Lepismatidae)". Journal of Pest Science. 79 (3): 155–161. doi:10.1007/s10340-006-0127-8. S2CID 39203675.
- "Diatomaceous Earth: Protect Food Storage". diatomaceousearth.com. Retrieved March 8, 2020.
- Capinera, John L. (2008). "Diatomaceous earth". In Capinera, John L. (ed.). Encyclopedia of Entomology (Second ed.). Springer. p. 1216. ISBN 978-1-4020-6242-1.
- "Pesticide Labeling Questions & Answers - Advertising Claims". EPA. Archived from the original on May 30, 2013. Retrieved July 7, 2013.
- Flynn, Thomas M. "Cryogenic Equipment and Cryogenic Systems Analysis." Cryogenic Engineering. Boca Raton etc.: CRC, 2005. Print.
- Nishimura, Shigeo (2001). Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis (1st ed.). New York: Wiley-Interscience. pp. 2–5. ISBN 9780471396987.
- "Prevention and Management of Insects and Mites in Farm-Stored Grain". Province of Manitoba. Archived from the original on October 18, 2013. Retrieved July 7, 2013.
- "21 CFR 573.340 - Diatomaceous earth" (PDF). Code of Federal Regulations (annual edition)—Title 21 - Food and Drugs—Part 573 - Food additives permitted in feed and drinking water of animals—Section 573.340 - Diatomaceous earth. Food and Drug Administration/U.S. Government Publishing Office. April 1, 2001. Retrieved February 9, 2016.
- "Diatomaceous Earth - How To Rid Bed Bugs Naturally - Organic". www.fertilizeronline.com. Retrieved April 17, 2022.
- "Diatomaceous Earth (DE)".
- Ferraz, E.; Coroado, J.; Silva, J.; Gomes, C.; Rocha, F. (2011). "Manufacture of ceramic bricks using recycled brewing spent kieselguhr". Materials and Manufacturing Processes. 26 (10): 1319–1329. doi:10.1080/10426914.2011.551908. S2CID 135734681.
- Reka, Arianit A.; Pavlovski, Blagoj; Makreski, Petre (October 2017). "New optimized method for low-temperature hydrothermal production of porous ceramics using diatomaceous earth". Ceramics International. 43 (15): 12572–12578. doi:10.1016/j.ceramint.2017.06.132.
- Bidle, Kay D.; Azam, Farooq (February 1999). "Accelerated dissolution of diatom silica by marine bacterial assemblages". Nature. 397 (6719): 508–512. Bibcode:1999Natur.397..508B. doi:10.1038/17351. S2CID 4397909.
- Zakharova, Yulia R.; Galachyants, Yuri P.; Kurilkina, Maria I.; Likhoshvay, Alexander V.; Petrova, Darya P.; Shishlyannikov, Sergey M.; Ravin, Nikolai V.; Mardanov, Andrey V.; Beletsky, Alexey V.; Likhoshway, Yelena V.; Mormile, Melanie R. (April 1, 2013). "The Structure of Microbial Community and Degradation of Diatoms in the Deep Near-Bottom Layer of Lake Baikal". PLOS ONE. 8 (4): e59977. Bibcode:2013PLoSO...859977Z. doi:10.1371/journal.pone.0059977. PMC 3613400. PMID 23560063.
- Washington, R.; Todd, M. C.; Lizcano, G.; Tegen, I.; Flamant, C.; et al. (2006). "Links between topography, wind, deflation, lakes and dust: The case of the Bodélé Depression, Chad" (PDF). Geophysical Research Letters. 33 (9): L09401. Bibcode:2006GeoRL..33.9401W. doi:10.1029/2006GL025827. ISSN 0094-8276. S2CID 14122607.
- "NIOSH 1988 OSHA PEL Project Documentation: List by Chemical Name: SILICA, AMORPHO". CDC. September 19, 2018.
- "Diatomaceous Earth: Its Use and Precautions" (PDF). Archived from the original (PDF) on July 17, 2013. Retrieved November 9, 2013.
- "Occupational Health Guideline for Amorphous Silica" (PDF). CDC. September 1978. Archived (PDF) from the original on March 12, 2020. Retrieved March 24, 2020.
- Bhadriraju Subramanyam; Rennie Roesli (July 10, 2003). "Inert Dusts" (PDF). Archived from the original (PDF) on July 10, 2003.
- "NIOSH Pocket Guide to Chemical Hazards - Silica, amorphous". CDC. Retrieved November 21, 2015.
- Hughes, Janet M.; Weill, Hans; Checkoway, Harvey; Jones, Robert N.; Henry, Melanie M.; Heyer, Nicholas J.; Seixas, Noah S.; Demers, Paul A. (1998). "Radiographic Evidence of Silicosis Risk in the Diatomaceous Earth Industry". American Journal of Respiratory and Critical Care Medicine. 158 (3): 807–814. doi:10.1164/ajrccm.158.3.9709103. ISSN 1073-449X. PMID 9731009.
External links
- International Chemical Safety Card 0248
- CDC – NIOSH Pocket Guide to Chemical Hazards
- Diatomite: Statistics and Information – USGS
- Tripolite: Tripolite mineral data Citat: "...A diatomaceous earth consisting of opaline silica..."
- Diatomaceous Earth: A Non Toxic Pesticide