Laparoscopy
Laparoscopy (from Ancient Greek λαπάρα (lapára) 'flank, side', and σκοπέω (skopéō) 'to see') is an operation performed in the abdomen or pelvis using small incisions (usually 0.5–1.5 cm) with the aid of a camera. The laparoscope aids diagnosis or therapeutic interventions with a few small cuts in the abdomen.[1]
| Laparoscopy | |
|---|---|
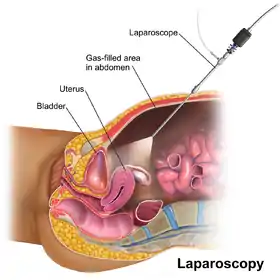 Illustration of Laparoscopy | |
| ICD-9-CM | 54.21 |
| MeSH | D010535 |
| OPS-301 code | 1-694 |
Laparoscopic surgery, also called minimally invasive procedure, bandaid surgery, or keyhole surgery, is a modern surgical technique. There are a number of advantages to the patient with laparoscopic surgery versus an exploratory laparotomy. These include reduced pain due to smaller incisions, reduced hemorrhaging, and shorter recovery time. The key element is the use of a laparoscope, a long fiber optic cable system that allows viewing of the affected area by snaking the cable from a more distant, but more easily accessible location.
Laparoscopic surgery includes operations within the abdominal or pelvic cavities, whereas keyhole surgery performed on the thoracic or chest cavity is called thoracoscopic surgery. Specific surgical instruments used in laparoscopic surgery include obstetrical forceps, scissors, probes, dissectors, hooks, and retractors. Laparoscopic and thoracoscopic surgery belong to the broader field of endoscopy. The first laparoscopic procedure was performed by German surgeon Georg Kelling in 1901.
Types of laparoscopes
There are two types of laparoscope:[2]
- A telescopic rod lens system, usually connected to a video camera (single-chip or three-chip)
- A digital laparoscope where a miniature digital video camera is placed at the end of the laparoscope, eliminating the rod lens system
The mechanism mentioned in the second type is mainly used to improve the image quality of flexible endoscopes, replacing conventional fiberscopes. Nevertheless, laparoscopes are rigid endoscopes. Rigidity is required in clinical practice. The rod-lens-based laparoscopes dominate overwhelmingly in practice, due to their fine optical resolution (50 µm typically, dependent on the aperture size used in the objective lens), and the image quality can be better than that of the digital camera if necessary. The second type of laparoscope is very rare in the laparoscope market and in hospitals.
Also attached is a fiber optic cable system connected to a "cold" light source (halogen or xenon) to illuminate the operative field, which is inserted through a 5 mm or 10 mm cannula or trocar. The abdomen is usually insufflated with carbon dioxide gas. This elevates the abdominal wall above the internal organs to create a working and viewing space. CO2 is used because it is common to the human body and can be absorbed by tissue and removed by the respiratory system. It is also non-flammable, which is important because electrosurgical devices are commonly used in laparoscopic procedures.[3]
Procedures
Patient position
During the laparoscopic procedure, the position of the patient is either in Trendelenburg position or in reverse Trendelenburg. These positions have an effect on cardiopulmonary function. In Trendelenburg's position, there is an increased preload due to an increase in the venous return from lower extremities. This position results in cephalic shifting of the viscera, which accentuates the pressure on the diaphragm. In the case of reverse Trendelenburg position, pulmonary function tends to improve as there is a caudal shifting of viscera, which improves tidal volume by a decrease in the pressure on the diaphragm. This position also decreases the preload on the heart and causes a decrease in the venous return leading to hypotension. The pooling of blood in the lower extremities increases the stasis and predisposes the patient to develop deep vein thrombosis (DVT).[4]
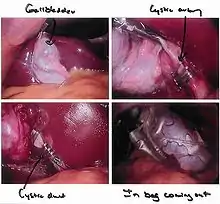
Gallbladder
Rather than a minimum 20 cm incision as in traditional (open) cholecystectomy, four incisions of 0.5–1.0 cm, or more recently, a single incision of 1.5–2.0 cm,[5] will be sufficient to perform a laparoscopic removal of a gallbladder. Since the gallbladder is similar to a small balloon that stores and releases bile, it can usually be removed from the abdomen by suctioning out the bile and then removing the deflated gallbladder through the 1 cm incision at the patient's navel. The length of postoperative stay in the hospital is minimal, and same-day discharges are possible in cases of early morning procedures.
Colon and kidney
In certain advanced laparoscopic procedures, where the specimen removed is too large to pull through a trocar site (as is done with gallbladders), an incision larger than 10 mm must be made. The most common of these procedures are removal of all or part of the colon (colectomy), or removal of the kidney (nephrectomy). Some surgeons perform these procedures completely laparoscopically, making the larger incision toward the end of the procedure for specimen removal, or, in the case of a colectomy, to also prepare the remaining healthy bowel to be reconnected (create an anastomosis). Many other surgeons feel that since they will have to make a larger incision for specimen removal anyway, they might as well use this incision to have their hand in the operative field during the procedure to aid as a retractor, dissector, and to be able to feel differing tissue densities (palpate), as they would in open surgery. This technique is called hand-assist laparoscopy. Since they will still be working with scopes and other laparoscopic instruments, CO2 will have to be maintained in the patient's abdomen, so a device known as a hand access port (a sleeve with a seal that allows passage of the hand) must be used. Surgeons who choose this hand-assist technique feel it reduces operative time significantly versus the straight laparoscopic approach. It also gives them more options in dealing with unexpected adverse events (e.g., uncontrolled bleeding) that may otherwise require creating a much larger incision and converting to a fully open surgical procedure.[6]
Conceptually, the laparoscopic approach is intended to minimise post-operative pain and speed up recovery times, while maintaining an enhanced visual field for surgeons. Due to improved patient outcomes, in the last two decades, laparoscopic surgery has been adopted by various surgical sub-specialties, including gastrointestinal surgery (including bariatric procedures for morbid obesity), gynecologic surgery, and urology. Based on numerous prospective randomized controlled trials, the approach has proven to be beneficial in reducing post-operative morbidities such as wound infections and incisional hernias (especially in morbidly obese patients), and is now deemed safe when applied to surgery for cancers such as cancer of colon.[7][8]

The restricted vision, the difficulty in handling of the instruments (new hand-eye coordination skills are needed), the lack of tactile perception, and the limited working area are factors adding to the technical complexity of this surgical approach. For these reasons, minimally invasive surgery has emerged as a highly competitive new sub-specialty within various fields of surgery. Surgical residents who wish to focus on this area of surgery gain additional laparoscopic surgery training during one or two years of fellowship after completing their basic surgical residency. In OB-GYN residency programs, the average laparoscopy-to-laparotomy quotient (LPQ) is 0.55.
In veterinary medicine
Laparoscopic techniques have also been developed in the field of veterinary medicine. Due to the relatively high cost of the equipment required, it has not become commonplace in most traditional practices today but rather limited to specialty practices. Many of the same surgeries performed in humans can be applied to animal cases – everything from an egg-bound tortoise to a German Shepherd can benefit from MIS. A paper published in JAVMA (Journal of the American Veterinary Medical Association) in 2005 showed that dogs spayed laparoscopically experienced significantly less pain (65%) than those that were spayed with traditional "open" methods.[9] Arthroscopy, thoracoscopy, and cystoscopy are all performed in veterinary medicine today.
Advantages
There are a number of advantages to the patient with laparoscopic surgery versus an open procedure. These include:
- Reduced hemorrhaging, which reduces the chance of needing a blood transfusion.[10][11]
- Smaller incision, which reduces pain and shortens recovery time, as well as resulting in less post-operative scarring.[11][12][13]
- Less pain, leading to less pain medication needed.[14][13]
- Use of regional anesthesia (with the recommendation of using a combined spinal and epidural anaesthesia) for laparoscopic surgery, as opposed to general anesthesia required for many non-laparoscopic procedures, can produce fewer complications and quicker recovery.[15]
- Although procedure times are usually slightly longer, hospital stay is less, and often with a same day discharge which leads to a faster return to everyday living.[12][16]
- Reduced exposure of internal organs to possible external contaminants, thereby reduced risk of acquiring infections.[7]
Although laparoscopy in adults is widely accepted, its advantages in children are questioned.[17][18] Benefits of laparoscopy appear to recede with younger age. Efficacy of laparoscopy is inferior to open surgery in certain conditions such as pyloromyotomy for infantile hypertrophic pyloric stenosis. Although laparoscopic appendectomy has less wound problems than open surgery, the former is associated with more intra-abdominal abscesses.[19]
Disadvantages
While laparoscopic surgery is clearly advantageous in terms of patient outcomes, the procedure is more difficult from the surgeon's perspective when compared to conventional, open surgery:
- Laparoscopic surgery requires pneumoperitoneum for adequate visualization and operative manipulation.[4]
- The surgeon has a limited range of motion at the surgical site, resulting in a loss of dexterity.[20]
- Poor depth perception.[20]
- Surgeons must use tools to interact with tissue rather than manipulate it directly with their hands. This results in an inability to accurately judge how much force is applied to tissue and higher risk of damaging tissue by applying more force than necessary. This limitation also reduces tactile sensation, making it more difficult for the surgeon to feel tissue (sometimes an important diagnostic tool, such as when palpating for tumors) and making delicate operations such as tying sutures more difficult.[21]
- The tool endpoints move in the opposite direction to the surgeon's hands due to the pivot point, making laparoscopic surgery a non-intuitive motor skill that is difficult to learn. This is called the fulcrum effect.[22]
- Some surgeries (carpal tunnel for instance) generally turn out better for the patient when the area can be opened up, allowing the surgeon to see the surrounding physiology, to better address the issue at hand. In this regard, keyhole surgery can be a disadvantage.[23]
Risks
Some of the risks are briefly described below:
- The major problems during laparoscopic surgery are related to the cardiopulmonary effect of pneumoperitoneum, systemic carbon dioxide absorption, venous gas embolism, unintentional injuries to intra-abdominal structures and patient positioning.[4]
- The most significant risks are from trocar injuries during insertion into the abdominal cavity, as the trocar is typically inserted blindly. Injuries include abdominal wall hematoma, umbilical hernias, umbilical wound infection, and penetration of blood vessels or small or large bowel.[24] The risk of such injuries is increased in patients who have a low body mass index[25] or have a history of prior abdominal surgery. While these injuries are rare, significant complications can occur, and they are primarily related to the umbilical insertion site. Vascular injuries can result in hemorrhage that may be life-threatening. Injuries to the bowel can cause a delayed peritonitis. It is very important that these injuries be recognized as early as possible.[26]
- In oncologic laparoscopic procedures there is a risk of port site metastases, especially in patients with peritoneal carcinomatosis. This incidence of iatrogenic dissemination of cancer might be reduced with special measures as trocar site protection and midline placement of trocars.[27]
- Some patients have sustained electrical burns unseen by surgeons who are working with electrodes that leak current into surrounding tissue. The resulting injuries can result in perforated organs and can also lead to peritonitis.[28]
- About 20% of patients undergo hypothermia during surgery and peritoneal trauma due to increased exposure to cold, dry gases during insufflation. The use of surgical humidification therapy, which is the use of heated and humidified CO2 for insufflation, has been shown to reduce this risk.[29]
- Not all of the CO
2 introduced into the abdominal cavity is removed through the incisions during surgery. Gas tends to rise, and when a pocket of CO2 rises in the abdomen, it pushes against the diaphragm (the muscle that separates the abdominal from the thoracic cavities and facilitates breathing), and can exert pressure on the phrenic nerve. This produces a sensation of pain that may extend to the patient's shoulders in about 80% of women for example. In all cases, the pain is transient, as the body tissues will absorb the CO2 and eliminate it through respiration.[30] - Coagulation disorders and dense adhesions (scar tissue) from previous abdominal surgery may pose added risk for laparoscopic surgery and are considered relative contra-indications for this approach.
- Intra-abdominal adhesion formation is a risk associated with both laparoscopic and open surgery and remains a significant, unresolved problem.[31] Adhesions are fibrous deposits that connect tissue to organ post surgery. Generally, they occur in 50-100% of all abdominal surgeries,[31] with the risk of developing adhesions the same for both procedures.[32][33] Complications of adhesions include chronic pelvic pain, bowel obstruction, and female infertility. In particular, small bowel obstruction poses the most significant problem.[32] The use of surgical humidification therapy during laparoscopic surgery may minimise the incidence of adhesion formation.[34] Other techniques to reduce adhesion formation include the use of physical barriers such as films or gels, or broad-coverage fluid agents to separate tissues during healing following surgery.[32]
- The gas used to make space and the smoke generated during surgical procedures can leak into the operating room through or around access devices as well as instruments. The gas plume can pollute the airspace shared by the operating team and patient with particles and potentially pathogens, including viral particles.[35][36]
Robotic laparoscopic surgery

In recent years, electronic tools have been developed to aid surgeons. Some of the features include:
- Visual magnification — use of a large viewing screen improves visibility
- Stabilization — Electromechanical damping of vibrations, due to machinery or shaky human hands
- Simulators — use of specialized virtual reality training tools to improve physicians' proficiency in surgery[37]
- Reduced number of incisions[38]
Robotic surgery has been touted as a solution to underdeveloped nations, whereby a single central hospital can operate several remote machines at distant locations. The potential for robotic surgery has had a strong military interest as well, with the intention of providing mobile medical care while keeping trained doctors safe from battle.
In January 2022, a robot performed the first ever successful laparoscopic surgery without the help of a human. The robot performed the surgery on the soft tissue of a pig. It succeeded at intestinal anastomosis, a procedure that involves connecting two ends of an intestine. The robot, named the Smart Tissue Autonomous Robot (STAR), was designed by a team of Johns Hopkins University researchers.[39]
Non-robotic hand-guided assistance systems
There are also user-friendly nonrobotic assistance systems that are single-hand guided devices with a high potential to save time and money. These assistance devices are not bound by the restrictions of common medical robotic systems. The systems enhance the manual possibilities of the surgeon and his/her team, regarding the need of replacing static holding force during the intervention.[40]
With laparoscopy providing tissue diagnosis and helping to achieve the final diagnosis without any significant complication and less operative time, it can be safely concluded that diagnostic laparoscopy is a safe, quick, and effective adjunct to non‑surgical diagnostic modalities, for establishing a conclusive diagnosis, but whether it will replace imaging studies as a primary modality for diagnosis needs more evidence.[41]
History

It is difficult to credit one individual with the pioneering of the laparoscopic approach. In 1901, Georg Kelling of Dresden, Germany, performed the first laparoscopic procedure in dogs, and, in 1910, Hans Christian Jacobaeus of Sweden performed the first laparoscopic operation in humans.[42]
In the ensuing several decades, numerous individuals refined and popularized the approach further for laparoscopy. The advent of computer chip-based television cameras was a seminal event in the field of laparoscopy. This technological innovation provided the means to project a magnified view of the operative field onto a monitor and, at the same time, freed both the operating surgeon's hands, thereby facilitating performance of complex laparoscopic procedures.
The first publication on modern diagnostic laparoscopy by Raoul Palmer appeared in 1947,[43] followed by the publication of Hans Frangenheim and Kurt Semm, who both practised CO
2 hysteroscopy from the mid-1970s.[44]
Patrick Steptoe, one of the pioneers of IVF, was important in popularizing laparoscopy in the UK. He published a textbook, Laparoscopy in Gynaecology, in 1967.[45]
In 1972, H. Courtenay Clarke invented, published, patented, presented, and recorded on film laparoscopic surgery, with instruments he invented and were marketed by the Ven Instrument Company of Buffalo, New York.[46] He was the first to perform a surgical laparoscopic process with standard sutures[47] and simple instruments This was meant to facilitate the application of laparoscopic surgery to all economic sectors by avoiding expensive materials and devices.[48]
In 1975, Tarasconi, from the Department of Ob-Gyn of the University of Passo Fundo Medical School (Passo Fundo, RS, Brazil), started his experience with organ resection by laparoscopy (Salpingectomy), first reported in the Third AAGL Meeting, Hyatt Regency Atlanta, November 1976 and later published in The Journal of Reproductive Medicine in 1981.[49] This laparoscopic surgical procedure was the first laparoscopic organ resection reported in medical literature.
In 1981, Semm, from the gynecological clinic of Kiel University, Germany, performed the first laparoscopic appendectomy. Following his lecture on laparoscopic appendectomy, the president of the German Surgical Society wrote to the Board of Directors of the German Gynecological Society suggesting suspension of Semm from medical practice. Subsequently, Semm submitted a paper on laparoscopic appendectomy to the American Journal of Obstetrics and Gynecology, at first rejected as unacceptable for publication on the grounds that the technique reported on was "unethical," but finally published in the journal Endoscopy. The abstract of his paper on endoscopic appendectomy can be found at the journal site.[44][50]
Semm established several standard procedures that were regularly performed, such as ovarian cyst enucleation, myomectomy, treatment of ectopic pregnancy and finally laparoscopic-assisted vaginal hysterectomy (also termed cervical intra-fascial Semm hysterectomy). He also developed a medical instrument company Wisap in Munich, Germany, which still produces various endoscopic instruments. In 1985, he constructed the pelvi-trainer = laparo-trainer, a practical surgical model whereby colleagues could practice laparoscopic techniques. Semm published over 1000 papers in various journals. He also produced over 30 endoscopic films and more than 20,000 colored slides to teach and inform interested colleagues about his technique. His first atlas, More Details on Pelviscopy and Hysteroscopy was published in 1976, a slide atlas on pelviscopy, hysteroscopy, and fetoscopy in 1979, and his books on gynecological endoscopic surgery in German, English, and many other languages in 1984, 1987, and 2002.[44]
In 1985, Erich Mühe, professor of surgery in Germany, performed the first laparoscopic cholecystectomy.[51] Afterward, laparoscopy gained rapid acceptance for non-gynecologic applications.
In 1987, Alfred Cuschieri performed the first minimally invasive surgery in the UK with his team at Ninewells Hospital after working with multiple researchers from across the world, including Patrick Steptoe. Cuschieri took advantage of smaller cameras to perform operations with smaller cuts and shorter recovery times. After some controversy and patient deaths, new laparoscopic training centres were set up as most surgeons lacked the necessary specialised training to perform laparoscopic surgery. The first opened in Dundee in 1991 and became the Cuschieri Skills Centre at Ninewells Hospital in 2004. As of 2008, 40 specialist centres around the world base their laparoscopic training on the Cuschieri Skills Centre.[52]
Prior to Mühe, the only specialty performing laparoscopy on a widespread basis was gynecology, mostly for relatively short, simple procedures such as a diagnostic laparoscopy or tubal ligation. The introduction in 1990 of a laparoscopic clip applier with twenty automatically advancing clips (rather than a single load clip applier that would have to be taken out, reloaded and reintroduced for each clip application) made general surgeons more comfortable with making the leap to laparoscopic cholecystectomies ( gall bladder removal). On the other hand, some surgeons continue to use the single clip appliers as they save as much as $200 per case for the patient, detract nothing from the quality of the clip ligation, and add only seconds to case lengths. Both laparoscopy tubal ligations and cholecystectomies may be performed using suturing and tying, thus further reducing the expensive cost of single and multiclips (when compared to suture). Once again this may increase case lengths but costs are greatly reduced (ideal for developing countries) and widespread accidents of loose clips are eliminated.
The first transatlantic surgery performed was a laparoscopic gallbladder removal in 2001. Remote surgeries and robotic surgeries have since become more common and are typically laparoscopic procedures.
Gynecological diagnosis
In gynecology, diagnostic laparoscopy may be used to inspect the outside of the uterus, ovaries, and fallopian tubes, as, for example, in the diagnosis of female infertility.[53] Usually, one incision is placed near the navel and a second near the pubic hairline. A special type of laparoscope called a fertiloscope, which is modified for transvaginal application, can be used. A dye test may be performed to detect any blockage in the reproductive tract, wherein a dark blue dye is passed up through the cervix and is followed with the laparoscope through its passage out into the fallopian tubes to the ovaries.[1]
See also
- Arthroscopic surgery
- Invasiveness of surgical procedures
- Laparotomy, an older open surgery technique for operations on the abdomen
- Natural orifice translumenal endoscopic surgery (NOTES)
- Percutaneous (surgery)
- Revision weight loss surgery
- Single port laparoscopy
References
- MedlinePlus > Laparoscopy Archived 26 July 2011 at the Wayback Machine Update Date: 21 August 2009. Updated by: James Lee, MD // No longer valid
- Stephen W, Eubanks S, Lee L, Swanstrom LL, Soper NJ, eds. (2004). Mastery of Endoscopic and Laparoscopic Surgery (2nd ed.). Lippincott Williams & Wilkins. ISBN 978-0781744454.
- "Training in diagnostic laparoscopy". Gfmer.ch. Archived from the original on 14 July 2014. Retrieved 10 October 2013.
- Srivastava, Arati; Niranjan, Ashutosh (2010). "Secrets of safe laparoscopic surgery: Anaesthetic and surgical considerations". Journal of Minimal Access Surgery. Medknow. 6 (4): 91–4. doi:10.4103/0972-9941.72593. ISSN 0972-9941. PMC 2992667. PMID 21120064.
 Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Bhandarkar D, Mittal G, Shah R, Katara A, Udwadia TE (January 2011). "Single-incision laparoscopic cholecystectomy: How I do it?". Journal of Minimal Access Surgery. 7 (1): 17–23. doi:10.4103/0972-9941.72367. PMC 3002000. PMID 21197237.
- Kaban GK, Czerniach DR, Litwin DE, Litwin DE (2003). "Hand-assisted laparoscopic surgery". Surgical Technology International. 11: 63–70. PMID 12931285.
- Shabanzadeh DM, Sørensen LT (December 2012). "Laparoscopic surgery compared with open surgery decreases surgical site infection in obese patients: a systematic review and meta-analysis". Annals of Surgery. 256 (6): 934–45. doi:10.1097/SLA.0b013e318269a46b. PMID 23108128. S2CID 5286895.
- Ma Y, Yang Z, Qin H, Wang Y (December 2011). "A meta-analysis of laparoscopy compared with open colorectal resection for colorectal cancer". Medical Oncology. 28 (4): 925–33. doi:10.1007/s12032-010-9549-5. PMID 20458560. S2CID 24029741.
- Devitt CM, Cox RE, Hailey JJ (September 2005). "Duration, complications, stress, and pain of open ovariohysterectomy versus a simple method of laparoscopic-assisted ovariohysterectomy in dogs". Journal of the American Veterinary Medical Association. 227 (6): 921–7. doi:10.2460/javma.2005.227.921. PMID 16190590. S2CID 16702386.
- Wang S, Shi N, You L, Dai M, Zhao Y (December 2017). "Minimally invasive surgical approach versus open procedure for pancreaticoduodenectomy: A systematic review and meta-analysis". Medicine. 96 (50): e8619. doi:10.1097/MD.0000000000008619. PMC 5815671. PMID 29390259.
- Li H, Zheng J, Cai JY, Li SH, Zhang JB, Wang XM, Chen GH, Yang Y, Wang GS (November 2017). "VS open hepatectomy for hepatolithiasis: An updated systematic review and meta-analysis". World Journal of Gastroenterology. 23 (43): 7791–7806. doi:10.3748/wjg.v23.i43.7791. PMC 5703939. PMID 29209120.
- Yi X, Chen S, Wang W, Zou L, Diao D, Zheng Y, He Y, Li H, Luo L, Xiong W, Wan J (August 2017). "A Systematic Review and Meta-Analysis of Laparoscopic and Open Distal Pancreatectomy of Nonductal Adenocarcinomatous Pancreatic Tumor (NDACPT) in the Pancreatic Body and Tail". Surgical Laparoscopy, Endoscopy & Percutaneous Techniques. 27 (4): 206–219. doi:10.1097/SLE.0000000000000416. PMID 28520652. S2CID 13671079.
- Zhang CD, Chen SC, Feng ZF, Zhao ZM, Wang JN, Dai DQ (August 2013). "Laparoscopic versus open gastrectomy for early gastric cancer in Asia: a meta-analysis". Surgical Laparoscopy, Endoscopy & Percutaneous Techniques. 23 (4): 365–77. doi:10.1097/SLE.0b013e31828e3e6e. PMID 23917592. S2CID 36337725.
- Tan S, Wu G, Zhuang Q, Xi Q, Meng Q, Jiang Y, Han Y, Yu C, Yu Z, Li N (September 2016). "Laparoscopic versus open repair for perforated peptic ulcer: A meta analysis of randomized controlled trials". International Journal of Surgery. 33 Pt A: 124–32. doi:10.1016/j.ijsu.2016.07.077. PMID 27504848.
- Sukhminder Jit Singh Bajwa and Ashish Kulshrestha (2016). "Anaesthesia for laparoscopic surgery: General vs regional anaesthesia". J Minim Access Surg. 12 (1): 4–9. doi:10.4103/0972-9941.169952. PMC 4746973. PMID 26917912.
{{cite journal}}: CS1 maint: uses authors parameter (link) - Silecchia G, Campanile FC, Sanchez L, Ceccarelli G, Antinori A, Ansaloni L, Olmi S, Ferrari GC, Cuccurullo D, Baccari P, Agresta F, Vettoretto N, Piccoli M (September 2015). "Laparoscopic ventral/incisional hernia repair: updated Consensus Development Conference based guidelines [corrected]". Surgical Endoscopy. 29 (9): 2463–84. doi:10.1007/s00464-015-4293-8. PMID 26139480.
- Gosemann JH, Lange A, Zeidler J, Blaser J, Dingemann C, Ure BM, Lacher M (August 2016). "Appendectomy in the pediatric population-a German nationwide cohort analysis". Langenbeck's Archives of Surgery. 401 (5): 651–9. doi:10.1007/s00423-016-1430-3. PMID 27118213. S2CID 9331647.
- Kane TD, Brown MF, Chen MK (May 2009). "Position paper on laparoscopic antireflux operations in infants and children for gastroesophageal reflux disease. American Pediatric Surgery Association". Journal of Pediatric Surgery. 44 (5): 1034–40. doi:10.1016/j.jpedsurg.2009.01.050. PMID 19433194.
- Raveenthiran V (October 2010). "Pediatric laparoscopy: Facts and factitious claims". Journal of Indian Association of Pediatric Surgeons. 15 (4): 122–8. doi:10.4103/0971-9261.72434. PMC 2995935. PMID 21170193.
- Swanström (2014). Mastery of endoscopic and laparoscopic surgery. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health. ISBN 978-1-4511-7344-4. OCLC 889995746.
- Westebring-van der Putten EP, Goossens RH, Jakimowicz JJ, Dankelman J (2008). "Haptics in minimally invasive surgery--a review". Minimally Invasive Therapy & Allied Technologies. 17 (1): 3–16. doi:10.1080/13645700701820242. PMID 18270873. S2CID 5439192.
- Gallagher AG, McClure N, McGuigan J, Ritchie K, Sheehy NP (September 1998). "An ergonomic analysis of the fulcrum effect in the acquisition of endoscopic skills". Endoscopy. 30 (7): 617–20. doi:10.1055/s-2007-1001366. PMID 9826140.
- Rodriguez, Anthony, Carpal Tunnel Surgery in Review, Beklind, 2009p.234
- Mayol J, Garcia-Aguilar J, Ortiz-Oshiro E, De-Diego Carmona JA, Fernandez-Represa JA (June 1997). "Risks of the minimal access approach for laparoscopic surgery: multivariate analysis of morbidity related to umbilical trocar insertion". World Journal of Surgery. 21 (5): 529–33. doi:10.1007/PL00012281. PMID 9204743. S2CID 29945805.
- Mirhashemi R, Harlow BL, Ginsburg ES, Signorello LB, Berkowitz R, Feldman S (September 1998). "Predicting risk of complications with gynecologic laparoscopic surgery". Obstetrics and Gynecology. 92 (3): 327–31. doi:10.1016/S0029-7844(98)00209-9. PMID 9721764. S2CID 24631884.
- Fuller J, Scott W, Ashar B, Corrado J. "Laparoscopic Trocar Injuries". A report from a U.S. Food and Drug Administration (FDA) Center for Devices and Radiological Health (CDRH) Systematic Technology Assessment of Medical Products (STAMP) Committee. Archived from the original on 9 April 2007.
- Segura-Sampedro, Juan José; Morales-Soriano, Rafael; Pineño Flores, Cristina; Craus-Miguel, Andrea; Sugarbaker, Paul H. (13 March 2021). "Laparoscopy technique in the setting of peritoneal metastases to avoid port site relapse". Surgical Oncology. 37: 101543. doi:10.1016/j.suronc.2021.101543. ISSN 0960-7404. PMID 33773282. S2CID 232386740.
- Karadag MA, Cecen K, Demir A, Bagcioglu M, Kocaaslan R, Kadioglu TC (April 2015). "Gastrointestinal complications of laparoscopic/robot-assisted urologic surgery and a review of the literature". J Clin Med Res. 7 (4): 203–10. doi:10.14740/jocmr2090w. PMC 4330011. PMID 25699115.
- Dean, Meara; Ramsay, Robert; Heriot, Alexander; Mackay, John; Hiscock, Richard; Lynch, A. Craig (May 2017). "Warmed, humidified CO2 insufflation benefits intraoperative core temperature during laparoscopic surgery: A meta-analysis". Asian Journal of Endoscopic Surgery. Wiley. 10 (2): 128–136. doi:10.1111/ases.12350. ISSN 1758-5902. PMC 5484286. PMID 27976517.
- Kaloo, Philip; Armstrong, Sarah; Kaloo, Claire; Jordan, Vanessa (30 January 2019). "Interventions to reduce shoulder pain following gynaecological laparoscopic procedures". The Cochrane Database of Systematic Reviews. Wiley. 1: CD011101. doi:10.1002/14651858.cd011101.pub2. ISSN 1465-1858. PMC 6353625. PMID 30699235.
- Brüggmann D, Tchartchian G, Wallwiener M, Münstedt K, Tinneberg HR, Hackethal A (November 2010). "Intra-abdominal adhesions: definition, origin, significance in surgical practice, and treatment options". Deutsches Ärzteblatt International. 107 (44): 769–75. doi:10.3238/arztebl.2010.0769. PMC 2992017. PMID 21116396.
- Leon DeWilde R (2007). "Postoperative abdominal adhesions and their prevention in gynaecological surgery. Expert consensus position". Gynecological Surgery. 4 (3): 161–168. doi:10.1007/s10397-007-0338-x.
- Lower AM, Hawthorn RJ, Clark D, Boyd JH, Finlayson AR, Knight AD, Crowe AM (August 2004). "Adhesion-related readmissions following gynaecological laparoscopy or laparotomy in Scotland: an epidemiological study of 24 046 patients". Human Reproduction. 19 (8): 1877–85. doi:10.1093/humrep/deh321. PMID 15178659.
- Peng Y, Zheng M, Ye Q, Chen X, Yu B, Liu B (January 2009). "Heated and humidified CO2 prevents hypothermia, peritoneal injury, and intra-abdominal adhesions during prolonged laparoscopic insufflations". The Journal of Surgical Research. 151 (1): 40–7. doi:10.1016/j.jss.2008.03.039. PMID 18639246.
- Cahill, R. A.; Dalli, J.; Khan, M.; Flood, M.; Nolan, K. (2020). "Solving the problems of gas leakage at laparoscopy". The British Journal of Surgery. 107 (11): 1401–1405. doi:10.1002/bjs.11977 (inactive 5 September 2022). PMC 7461047. PMID 32856306.
{{cite journal}}: CS1 maint: DOI inactive as of September 2022 (link) - Limchantra, Ice V.; Fong, Yuman; Melstrom, Kurt A. (2019). "Surgical Smoke Exposure in Operating Room Personnel". JAMA Surgery. 154 (10): 960–967. doi:10.1001/jamasurg.2019.2515. PMID 31433468. S2CID 201116813.
- Ahmed K, Keeling AN, Fakhry M, Ashrafian H, Aggarwal R, Naughton PA, Darzi A, Cheshire N, Athanasiou T, Hamady M (January 2010). "Role of virtual reality simulation in teaching and assessing technical skills in endovascular intervention". Journal of Vascular and Interventional Radiology. 21 (1): 55–66. doi:10.1016/j.jvir.2009.09.019. PMID 20123191.
- Samarasekera D, Kaouk JH (July 2014). "Robotic single port surgery: Current status and future considerations". Indian Journal of Urology. Indian J Urol. 30 (3): 326–32. doi:10.4103/0970-1591.128504. PMC 4120222. PMID 25097321.
- Saeidi, H.; Opfermann, J. D.; Kam, M.; Wei, S.; Leonard, S.; Hsieh, M. H.; Kang, J. U.; Krieger, A. (26 January 2022). "Autonomous robotic laparoscopic surgery for intestinal anastomosis". Science Robotics. 7 (62): eabj2908. doi:10.1126/scirobotics.abj2908. PMC 8992572. PMID 35080901.
- Thai, Mai Thanh; Phan, Phuoc Thien; Hoang, Trung Thien; Wong, Shing; Lovell, Nigel H.; Do, Thanh Nho (2020). "Advanced Intelligent Systems for Surgical Robotics". Advanced Intelligent Systems. 2 (8). arXiv:2001.00285. doi:10.1002/aisy.201900138. S2CID 209531913.
- Nar AS, Bawa A, Mishra A, Mittal A (July 2014). "Role of Diagnostic Laparoscopy in Chronic Abdominal Conditions with Uncertain Diagnosis". Nigerian Journal of Surgery. 20 (2): 75–78. doi:10.4103/1117-6806.137301. PMC 4141449. PMID 25191097.
- Hatzinger, Martin; Kwon, S.T.; Langbein, S.; Kamp, S.; Häcker, Axel; Alken, Peter (2006). "Hans Christian Jacobaeus: Inventor of Human Laparoscopy and Thoracoscopy". Journal of Endourology. 20 (11): 848–850. doi:10.1089/end.2006.20.848. PMID 17144849.
- Palmer R (1947). "[Not Available]". Gynécologie et Obstétrique. 46 (4): 420–31. PMID 18917806.
- Bhattacharya K (January 2007). "Kurt Semm: A laparoscopic crusader". J Minim Access Surg. 3 (1): 35–6. doi:10.4103/0972-9941.30686. PMC 2910380. PMID 20668618.
- Edwards, R. G. (1 September 1996). "Patrick Steptoe, CBE, MBChB, D.Se., FRCS (Ed), FRCOG, FRS". Human Reproduction. 11 (Supplement_5): 215–234. doi:10.1093/humrep/11.suppl_5.215. ISSN 0268-1161. PMID 8968782.
- Clarke HC (April 1972). "Laparoscopy--new instruments for suturing and ligation". Fertility and Sterility. 23 (4): 274–7. doi:10.1016/S0015-0282(16)38886-0. PMID 4258561.
- Mishra, R. K. (2013). Textbook of Practical Laparoscopic Surgery. New Delhi: Jaypee Brothers Pvt. Ltd. p. 5. ISBN 978-93-5025-941-2. OCLC 870588241.
- Clarke, H. Courtenay (1972). "Laparoscopy—New Instruments for Suturing and Ligation". Fertility and Sterility. Elsevier BV. 23 (4): 274–277. doi:10.1016/s0015-0282(16)38886-0. ISSN 0015-0282. PMID 4258561.
- Tarasconi JC (October 1981). "Endoscopic salpingectomy". The Journal of Reproductive Medicine. 26 (10): 541–5. PMID 6458700.
- Semm K (March 1983). "Endoscopic appendectomy". Endoscopy. 15 (2): 59–64. doi:10.1055/s-2007-1021466. PMID 6221925.
- Litynski, G. S. (1998). "Erich Mühe and the Rejection of Laparoscopic Cholecystectomy (1985): A Surgeon Ahead of His Time". Journal of the Society of Laparoendoscopic Surgeons. 2 (4): 341–346. PMC 3015244. PMID 10036125.
- "Doctor who shrunk the surgeon". Times of Malta. Retrieved 17 May 2022.
- "Female Pelvic Laparoscopy". Mayo Clinic. Retrieved 22 September 2020.
External links
- Feder, Barnaby J. (17 March 2006). "Surgical Device Poses a Rare but Serious Peril". The New York Times.