Moor frog
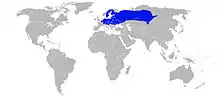
The moor frog (Rana arvalis) is a slim, reddish-brown, semiaquatic amphibian native to Europe and Asia. It is a member of the family Ranidae, or true frogs. The frog is known for its expansive range covering a significant portion of Eurasia. Male frogs are also known to develop temporary blue coloration for mating. The frog has an IUCN listing of Least Concern but it varies in some countries. France and Romania consider the frog nearly extinct and critically endangered, respectively.[2][3]
| Moor frog | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Amphibia |
| Order: | Anura |
| Family: | Ranidae |
| Genus: | Rana |
| Species: | R. arvalis |
| Binomial name | |
| Rana arvalis Nilsson, 1842 | |
 | |

Taxonomy
The family the moor frog belongs to, Ranidae, is a broad group containing 605 species. The family is like a “catch-all” for ranoid frogs that do not belong to any other families.[4] Since this is the case, the characteristics that define them are more general, and the frogs are found all throughout the world, on every continent but Antarctica.
The moor frog's genus, Rana, is a little more specific. Frogs of this genus are found in Europe and Asia. The moor frog is not found in the Americas.
The moor frog's scientific name, Rana arvalis means "frog of the fields". It is also called the Altai brown frog because frogs from the Altai Mountains in Asia have been included in the R. arvalis species. The Altai frogs have some different characteristics such as shorter shins, but currently there is no official distinction and all frogs are known as one species—Rana arvalis.[1] The taxonomy may be better defined in the future.
Description
The moor frog is a small bog frog, characterized by an unspotted belly, a large, dark ear spot, and — often, not always — a pale stripe down the center of the back. They are generally described as a reddish-brown, but can also be yellow, gray, or light olive. Their bellies are white or yellow and they have a "bandit-like" black stripe going from their nose to their ears. They vary from 5.5 to 6.0 cm long, but can reach up to 7.0 cm in length; their heads are more tapered than those of the Common frog (Rana temporaria). The skin on their flanks and thighs is smooth, and the posterior tongue is forked and free. They have horizontal pupils, their feet are partially webbed, and their back legs are shorter than those of other species of frogs. Males are different from females because of the presence of nuptial pads on their first fingers and their paired guttural vocal sacs.[5]
Distribution and habitat
The moor frog’s range covers a large majority of Europe and Asia. Its east-west range extends from northeastern France and northern Belgium all the way to the Lena River in Siberia. Its north-south range extends as far north as the 69th parallel in Finland — where the sun is visible for 24 hours during the summer solstice— and as far south as the Pannonian basin in Central Europe. The moor frog can be found in a wide range of altitudes. In the western region of its range the moor frog can be found as high up as 900 meters above sea level, and in the eastern portion the moor frog can be found as high up as 2000 meters in the Altai.[6] Within this geographical range the moor frog is often found in bodies of still water with littoral vegetation and pH below 6.[6][7] The diversity of habitats demonstrates the frog’s plasticity towards habitat.[6]
The types of land moor frogs can inhabit are greatly varied. They live in tundra, forest tundra, forest, forest steppe, steppe, forest edges and glades, semideserts, swamps, meadows, fields, bush lands, and gardens. They prefer areas untouched by humans, such as damp meadows and bogs, but they still may be able to live in agricultural and urban areas.[1]
Moor frogs provide a good model for studying local adaptation as they experience a wide range of environments and are relatively limited in their movements.[8][9] Their restriction in movements implies limited gene flow and facilitates evolution through adaptive genetic differentiation among populations.[10]
Furthermore, a historical reference was also found from the 13th century by Bartholomeus Anglicus ("Rana palustres").[11] The species has been successfully bred in captivity in the UK and a reintroduction has been proposed as part of Celtic Reptile & Amphibian's rewilding plans.[12][13][14]
Historical Distribution
The earliest fossil record of the moor frog extends back to between the Pliocene and Early Pleistocene found in Dvorníky-Včeláre, Slovakia.[15] Other fossil records of the moor frog from the early Pleistocene were found on land that is inside the modern range of the moor frog.[15] Fossil records from the middle Pleistocene demonstrate the range extended as far south as south-central France and as far west as the eastern coast of the Great Britain.[15] Records from the late Pleistocene show the range extended as far south as Bosnia and Herzegovina and Azerbaijan.[15]
Distribution in Romania
There are three main regions where the moor frog can be found in Romania. The first is the Transylvanian region which includes the Western Plains, the Transylvanian Plateau, and the Eastern Carpathians. The second region is northern part of Romanian Moldavia. The third and smallest region is the Tisa River Basin—north of Maramureș.[16] The largest Romanian population of moor frog lives in the Western Plains.[16] The population of moor frog in Romanian Moldavia is isolated from the populations in Transylvania.[16] Most populations of moor frog in Romania are isolated and not contiguous.[16]
Each population may have 200-400 adults; however, exceptional populations of 2000 adults have been found as well. Most Romanian populations of moor frog can be found between 108-414 meters above sea level.[16] Exceptional populations have been found to exist at 740 meters above sea level.[16]
Populations are isolated because of edge effects of human developments.[16] In Romania the moor frog is known to live in humid habitats that border land with human activity, such as flooded agricultural fields, ditches on the side of roads, small canals and streams, and human settlements.[16] The moor frog is sparingly found in habitats with little human activity. Swamps are one of the few habitats with little human activity that host moor frogs.[16]
Conservation
Population Threats
It is currently classified as Least Concern by the IUCN. However, the moor frog may soon be impacted by the destruction and pollution of breeding sites and adjacent habitats, mostly through urbanization, recreational use of waterside areas, and intensive agriculture. The species does not appear to be notably susceptible to chytridiomycosis, although the fungus has been detected in frogs in Germany.[1]
The 2009 IUCN Red List status of the moor frog does not properly reflect the current declining nature of the moor frog.[17] There is a general lack of research on the conservation status of the moor frog in many EU member states and in-range countries. However, a European Habitats Directive performed in 2013 revealed that 19 of the 28 member states of the time reported that conservation status of the moor frog were unfavorable. 11 of the 19 said that their status was in decline as well.[17] It is known that existing populations in Europe are small in number which indicate a significant loss of genetic diversity.[17] This lack of genetic diversity threatens the current stability of populations and long-term survival because of the increased risk of inbreeding.[17]
Conservation Status in France
The moor frog is considered nearly extinct in France where the western limit of the moor frog range extends. As of 2020, there are only four isolated populations in France. These four were once a contiguous metapopulation.[17] In France, moor frog habitats are limited and of poor quality due to significant human development that encroaches on and destroys moor frog habitats. Edge effects of human developments also fragment and degrade remaining habitats. Mild inbreeding greatly reduces the moor frog fitness due to the small number of individuals in these isolated populations,.[17]
Conservation Efforts
Acidification, eutrophication, and other forms of water pollution are negatively affecting the aquatic habitats of moor frogs which is exacerbating their already critical condition.[17] Moor frogs normally enjoy acidic environments; however, peat bogs which produce these acidic conditions have poor buffering properties that make them susceptible to drastic decreases of pH even below 4.5.[18][19] At pHs below 4.5, the moor frog can no longer reproduce.[18] There are various conservation practices being initiated in order to remediate these pH driven affects.[18] Liming of peat bogs by adding chalk can increase pH.[18] While this method may allow for moor frog reproduction to occur in the short-term, the effect is only temporary and acidification will ultimately reoccur.[18] Protection and addition of riparian zones by preventing grazing and replanting littoral vegetation aids the rewetting process of drained land.[18] Drainage of land for agriculture is especially dangerous to the moor frog because they are prone to desiccation.[18] Conservation efforts undertaken for the moor frog are most effective when executed in small scale phases.[18] These small scale phases are more easily managed and receive more attention.[18]
Diet
An adult moor frog’s diet consists of any mobile and terrestrial animals that they can physically ingest. Moor frogs most commonly consume beetles; however, other insects from the orders hemiptera (true bugs), hymenoptera, and diptera (flies) are consumed as well. Non-insect invertebrate of orders e.g. gastropoda (snails and slugs), arachnida, and myriapoda (centipedes and millipedes) are observed to be consumed by moor frogs.[19] Beetles make up the majority of the moor frog's diet due to their abundance. Large moor frogs do appear to have a preference for beetles because they are larger than most other insect prey. Large moor frogs tend to consume large prey and small moor frogs consume small prey.[20] This behavior is assumed to have evolved to reduce competition between moor frogs and/or to maximize net energy gained from feeding. Larger moor frogs consume fewer small insects not out of generosity towards smaller moor frogs, but because all large moor frogs were once small moor frogs. Thus, if large moor frogs consumed large and small prey indifferently there may not be enough small prey for smaller moor frogs harming the moor frog and its genes. Aside from size preferences, individual moor frogs do not appear to prefer more energetically favorable prey over less energetically favorable prey of equal size.[19] The moor frog will ingest any animal that it is able to swallow and in close proximity. Moor frogs are opportunistic predators that wait for prey to appear before consuming them;[19] as opposed to intentional predators that actively hunt for prey. More mobile prey are more often consumed by the moor frog because of their opportunistic nature.
Plant matter and inedible objects such as pebbles are also found to be consumed by the moor frog.[19] Plant matter is found to be consumed in greater quantities when more prey has been consumed which suggests that plant matter is consumed accidentally during the capture of prey.[21] The moor frog’s shed skin is also consumed; however, it is unknown whether consumption of shed skin is accidental or intentional in nature.[21]
Mating
Multimale amplexus is the predominant method of mating that the moor frog performs.[22] This suggests that post-copulatory competition may be just as important as pre-copulatory competition. The sperm of male moor frogs compete in the female reproductive tract for fertilization of the female's egg.
Female frogs do not appear to prefer males of a particular size.[22] Females did prefer to mate with males that have successfully helped produce offspring with that female in the past.[22]
Long thumb length suggests poor sperm quality, and short thumb length suggests greater sperm quality. Males with quality sperm bred progeny with greater chances of survival. Despite this correlation, female individuals did not appear to prefer thumb length or be able to detect variation in thumb length.[22]
Blue Coloration
Male moor frogs turn a conspicuous blue during its mating season but only for a few days during peak reproductive activity;[23] females remain brown during this time. While the blue is conspicuous to human vision, the greatest color change in male moor frogs occurs in the ultraviolet region from 350-450nm, invisible to human vision.[24] Again, this shift in reflectance does not appear in female moor frogs.
Males who have mated appeared bluer and were recorded as having higher body temperatures.[23][24] Males who have higher body temperatures also appeared bluer.[23] Change in reflectance could be a method of intrasexual communication that signal an individual’s sex. Males in a multimale amplexus (multiple males mate with a single female frog) will be able to more easily differentiate a frog’s sex if males are bright blue versus a brown female. Male moor frogs will not mount an individual if they have bright blue coloration. This is evolutionary advantageous because males that are able to differentiate coloration will be not be susceptible to wasting their sperm and time mistakenly mating with another male.
Blue reflectance may also be a form of intersexual communication.[24] It is hypothesized that males with brighter blue coloration may signal greater sexual and genetic fitness;[24] however, studies have only revealed tadpoles fathered by bright blue individuals had greater chances of survival when pitted against large beetle larvae than when fathered by dull individuals.[24]
Ecology
Hibernation

Moor frogs will hibernate sometime between September and June, depending on the latitude of the location. Frogs in southwestern, plain habitats will leave later (around November or December) and return earlier (February). However, frogs in cold, polar areas will disappear sooner (in September) and return later (in June).[25]
Breeding
The mating season takes place between March and June right after the end of hibernation. Males form breeding choruses, and their songs sound similar to those of the agile frog, (Rana dalmatina). Their calls can "sound like air escaping from a submerged empty bottle: 'waug...waug...waug'. Males can also develop bright-blue coloration for a few days during the season.[26]
The spawning happens very quickly and is completed in 3 to 28 days. The spawn of each frog is laid in one or two clusters of 500-3000 eggs in warm, shallow waters.
Metamorphosis
Metamorphosis happens between June and October. Larvae are about 45 mm long and dark in color with small dots. When the larvae transform into tadpoles, their diet consists of algae and small invertebrates. The adult frogs' feeding is halted during the breeding season, but their diets consist of insects and various invertebrates.
Environmental plasticity
Increased acidity levels in breeding areas may be problematic for moor frog populations, as it reduces survival and growth of the aquatic embryos and larvae.[27][28][29][30][31] When exposed to acidity, moor frogs were shown to be able to adapt relatively rapidly (within 16–40 generations). Local adaptation to acidity is also possible in survival during the embryonic stage, during which frogs are most sensitive to severe acidity.[32] Moreover, compared to those from neutral sites, acid origin populations have higher embryonic and larval acid tolerance (survival and larval period were less negatively affected by low pH), higher larval growth but slower larval development rates, and larger metamorphosing size. Divergence in embryonic acid tolerance and metamorphic size correlates most strongly with breeding pond pH, whereas divergence in larval period and larval growth correlates most strongly with latitude and predator density, respectively.[33]
Maternal effects
For female moor frogs, in order to optimize their maternal investments, they need to balance between their own fitness and the fitness of their offspring.[34][35] Additionally, under the great pressure exerted by acidification, female moor frogs also need to do a trade-off between quality and quantity of the offspring.[36]
Environmental stress, like acidity, may either select for or select against certain phenotypes and hence may increase variation in fitness. The adaptive optima for the population would shift greatly compared to those living in less acidic and more benign environments, thereby making the allocation of resources even more important. As a result of the increasing variation in fitness, frogs from acidic environments may also favor different reproductive strategies than those more benign environments.[37] Compared to neutral origin females, acid origin females tend to invest relatively more in fecundity than in egg size, invest more in their offspring than in self-maintenance, and increase their reproductive effort as their residual reproductive value decreases.[36] Consequently, acid origin females increase the clutch size and total reproductive output with age, while neutral origin females only increase egg size but not clutch size or total reproductive output with age.[36]
In conclusion, environmental acidification lowers maternal investment, selects for investment in larger eggs at a cost to fecundity, imposes negative effects on reproductive output, and alters the relationship between female phenotype and maternal investment, as well as strengthens the egg-size-fecundity trade-off.[36]
References
- Kuzmin, S.; Tarkhnishvili, D.; Ishchenko, V.; Tuniyev, B.; Beebee, T.; Anthony, B.P.; Schmidt, B.; Ogrodowczyk, A.; Ogielska, M.; Babik, W.; Vogrin, M.; Loman, J.; Cogalniceanu, D.; Kovács, T.; Kiss, I. (2016) [errata version of 2016 assessment]. "Rana arvalis". IUCN Red List of Threatened Species. 2009: e.T58548A86232114.
- SAS, ISTVÁN; COVACIU-MARCOV, SEVERUS-DANIEL; DEMETER, LÁSZLÓ; CICORT-LUCACIU, ALFRED-ŞTEFAN; STRUGARIU, ALEXANDRU (August 2008). "Distribution and status of the moor frog (Rana arvalis) in Romania" (PDF). Zeitschrift für Feldherpetologie: 337–354.
- Mergeay, Joachim; Neyrinck, Sabrina; Cox, Karen (2020). "Conservation Genetic status of Moor Frog in France" (PDF). Research Institute for Nature and Forest.
- Niles Eldredge, ed. (2002). Life on Earth: An Encyclopedia of Biodiversity, Ecology, and Evolution. Santa Barbara, California: ABC-CLIO.
- "Moor Frog (Rana arvalis)". World Association of Zoos and Aquariums. 2007.
- ROČEK, ZBYNĚK; ŠANDERA, MARTIN (August 2008). "Distribution of Rana arvalis in Europe: a historical perspective" (PDF). Zeitschrift für Feldherpetologie: 135–150.
- Mergeay, Joachim; Neyrinck, Sabrina; Cox, Karen (2020). "Conservation Genetic status of Moor Frog in France" (PDF). Research Institute for Nature and Forest.
- Ward, R. D.; Skibinski, D. O.; Woodwark, M. (1992). "Protein heterozygosity, protein structure, and taxonomic differentiation". In K. M. Hecht (ed.). Evolutionary biology. New York: Plenum press. pp. 73–159.
- Beebee, T. J. C. (1996). Ecology and conservation of amphibians. London: Chapman and Hall.
- Hendry, A. P.; Kinnison, M. T.; Day, T.; Taylor, E. B. (2001). "Population mixing and the adaptive divergence of quantitative traits in discrete populations: a theoretical framework for empirical tests". Evolution. 55 (3): 459–466. doi:10.1111/j.0014-3820.2001.tb00780.x. S2CID 23917769.
- Raye, Lee (October 2017). "Frogs in pre-industrial Britain". The Herpetological Journal – via British Herpetological Society.
- "Frog turns blue for first time in 700 years amid calls for rare amphibians to be reintroduced to Britain". The Telegraph.
{{cite web}}: CS1 maint: url-status (link) - "Blue Moor Frog Once Again Seen in the UK After 700 Years in Time for Mating Season".
{{cite web}}: CS1 maint: url-status (link) - "'Who doesn't love a turtle?' The teenage boys on a mission – to rewild Britain with reptiles". the Guardian. 2021-01-10. Retrieved 2021-10-27.
- ROČEK, ZBYNĚK; ŠANDERA, MARTIN (August 2008). "Distribution of Rana arvalis in Europe: a historical perspective" (PDF). Zeitschrift für Feldherpetologie: 135–150.
- SAS, ISTVÁN; COVACIU-MARCOV, SEVERUS-DANIEL; DEMETER, LÁSZLÓ; CICORT-LUCACIU, ALFRED-ŞTEFAN; STRUGARIU, ALEXANDRU (August 2008). "Distribution and status of the moor frog (Rana arvalis) in Romania" (PDF). Zeitschrift für Feldherpetologie: 337–354.
- Mergeay, Joachim; Neyrinck, Sabrina; Cox, Karen (2020). "Conservation Genetic status of Moor Frog in France" (PDF). Research Institute for Nature and Forest.
- VAN DELFT, JEROEN; CREEMERS, RAYMOND (August 2008). "Distribution, status and conservation of the moor frog (Rana arvalis) in the Netherlands". Zeitschrift für Feldherpetologie: 255–268 – via Google Scholar.
- Stojanova, Anelia; Mollov, Ivelin (2008-11-01). "DIET AND TROPHIC NICHE OVERLAP OF THE MOOR FROG (Rana arvalis Nilsson, 1842) AND THE COMMON FROG (Rana temporaria L., 1758) FROM POLAND". Proceedings of the Anniversary Scientific Conference of Ecology: 181–190 – via Google Scholar.
- LOW, PETER; TOROK, JANOS (July 1998). "Prey size selection and food habits of Water Frogs and Moor Frogs from Kis-Balaton, Hungary" (PDF). Herpetozoa: 71–78 – via Google Scholar.
- ASZALÓS, Lilla; BOGDAN, Horia; KOVÁCS, Éva-Hajnalka; PETER, Violeta-Ionela (12 March 2005). "Food composition of two Rana species on a forest habitat (Livada Plain, Romania)" (PDF). North-Western Journal of Zoology. 1: 25–30 – via Google Scholar.
- Sherman, Craig; Sagvik, Jorgen; Olsson, Mats (October 26, 2010). "Female Choice for Males with Greater Fertilization Success in the Swedish Moor Frog, Rana arvalis". PLOS ONE. 5 (10): e13634. doi:10.1371/journal.pone.0013634. PMC 2964304. PMID 21049015.
- Hettyey, Attila; Herczeg, Gabor; Laurila, Anssi; Crochet, Pierre-Andre; Merilä, Juha (2009-01-01). "Body temperature, size, nuptial colouration and mating success in male Moor Frogs (Rana arvalis)". Amphibia-Reptilia. 30: 37–43. doi:10.1163/156853809787392784 – via Google Scholar.
- Ries, C; Spaethe, J; Sztatecsny, M; Strondl, C; Hödl, W (20 October 2008). "Turning blue and ultraviolet: sex-specific colour change during the mating season in the Balkan moor frog". Journal of Zoology. 276 (3): 229–236. doi:10.1111/j.1469-7998.2008.00456.x – via Google Scholar.
- Kuzmin, Sergius L. (1999). "Rana arvalis". AmphibiaWeb. Retrieved 26 March 2009.
- Otto Berninghausen; Friedo Berninghausen. "Moor frog - Rana arvalis".
- Freda, J. (1986). "The influence of acidic pond water on amphibians: a review". Water, Air, & Soil Pollution. 30 (1–2): 439–450. Bibcode:1986WASP...30..439F. doi:10.1007/bf00305213. S2CID 189826247.
- Freda, J.; Sadinski, W. J.; Dunson, W. A. (1991). "Long term monitoring of amphibian populations with respect to the effects of acidic deposition". Water, Air, & Soil Pollution. 55 (3–4): 445–462. Bibcode:1991WASP...55..445F. doi:10.1007/bf00211205. S2CID 85373859.
- Pierce, B. A. (1985). "Acid tolerance in amphibians". BioScience. 35 (4): 239–243. doi:10.2307/1310132. JSTOR 1310132.
- Pierce, B. A. (1993). "Effects of acid precipitation on amphibians". Ecotoxicology. 2 (1): 65–77. doi:10.1007/bf00058215. PMID 24203120. S2CID 5050232.
- Böhmer, J.; Rahmann, H. (1990). "Influence of surface water acidification on amphibians". In W. Hanke (ed.). Biology and physiology of Amphibians. Stuttgart: Gustav Fischer Verlag.
- Räsänen, K.; Laurila, A.; Merilä, J. (2003). "Geographic variation in acid stress tolerance of the moor frog Rana arvalis. I. Local adaptation". Evolution. 57 (2): 352–362. doi:10.1554/0014-3820(2003)057[0352:gviast]2.0.co;2. S2CID 42580879.
- Hangartner, S; Laurila, A; Räsänen, K (2012a). "Adaptive divergence in moor frog (Rana Arvalis) populations along an acidification gradient: inferences from Qst-Fst correlations". Evolution. 66 (3): 867–881. doi:10.1111/j.1558-5646.2011.01472.x. PMID 22380445. S2CID 20964432.
- Roff, D. A. 1992. "The evolution of life histories. Local adaptation and genetics of acid-stress" Hall, New York, New York, USA.
- Sinervo, B (1999). "Mechanistic analysis of natural selection and a refinement of Lack's and Williams's principles". American Naturalist. 154 (S1): S26–S42. doi:10.2307/2463885. JSTOR 2463885. PMID 29586707.
- Räsänen, K.; Söderman, F.; Laurila, A.; Merilä, J. (2008). "Geographic variation in maternal investment: acidity affects egg size and fecundity in Rana arvalis". Ecology. 89 (9): 2553–2562. doi:10.1890/07-0168.1. PMID 18831176.
- Kisdi, E., G. Meszna, and L. Pasztor. 19 1998. "Individual optimization: mechanisms shaping the optimal reaction norm". Evolutionary Ecology 12:211-221.
- Some parts of this article were translated from the article Grenouille des champs on the French language Wikipedia.
