Seagrass
Seagrasses are the only flowering plants which grow in marine environments. There are about 60 species of fully marine seagrasses which belong to four families (Posidoniaceae, Zosteraceae, Hydrocharitaceae and Cymodoceaceae), all in the order Alismatales (in the clade of monocotyledons).[1] Seagrasses evolved from terrestrial plants which recolonised the ocean 70 to 100 million years ago.
| Seagrasses Temporal range: | |
|---|---|
 | |
| Zostera marina – the most abundant seagrass species in the Northern Hemisphere | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Tracheophytes |
| Clade: | Angiosperms |
| Clade: | Monocots |
| Order: | Alismatales R.Br. ex Bercht. & J.Presl |
| Families | |
|
See Taxonomy | |
The name seagrass stems from the many species with long and narrow leaves, which grow by rhizome extension and often spread across large "meadows" resembling grassland; many species superficially resemble terrestrial grasses of the family Poaceae.
Like all autotrophic plants, seagrasses photosynthesize, in the submerged photic zone, and most occur in shallow and sheltered coastal waters anchored in sand or mud bottoms. Most species undergo submarine pollination and complete their life cycle underwater. While it was previously believed this pollination was carried out without pollinators and purely by sea current drift, this has been shown to be false for at least one species, Thalassia testudinum, which carries out a mixed biotic-abiotic strategy. Crustaceans (such as crabs, Majidae zoae, Thalassinidea zoea) and syllid polychaete worm larvae have both been found with pollen grains, the plant producing nutritious mucigenous clumps of pollen to attract and stick to them instead of nectar as terrestrial flowers do.[2]
Seagrasses form dense underwater seagrass meadows which are among the most productive ecosystems in the world. They function as important carbon sinks[3] and provide habitats and food for a diversity of marine life comparable to that of coral reefs.
Overview
Seagrasses are a paraphyletic group of marine angiosperms which evolved in parallel three to four times from land plants back to the sea. The following characteristics can be used to define a seagrass species. It lives in an estuarine or in the marine environment, and nowhere else. The pollination takes place underwater with specialized pollen. The seeds which are dispersed by both biotic and abiotic agents are produced underwater.[4] The seagrass species have specialized leaves with a reduced cuticle, an epidermis which lacks stomata and is the main photosynthetic tissue. The rhizome or underground stem is important in anchoring. The roots can live in an anoxic environment and depend on oxygen transport from the leaves and rhizomes but are also important in the nutrient transfer processes.[5][4]
Seagrasses profoundly influence the physical, chemical, and biological environments of coastal waters.[4] Though seagrasses provide invaluable ecosystem services by acting as breeding and nursery ground for a variety of organisms and promote commercial fisheries, many aspects of their physiology are not well investigated. Several studies have indicated that seagrass habitat is declining worldwide.[6][7] Ten seagrass species are at elevated risk of extinction (14% of all seagrass species) with three species qualifying as endangered. Seagrass loss and degradation of seagrass biodiversity will have serious repercussions for marine biodiversity and the human population that depends upon the resources and ecosystem services that seagrasses provide.[8][4]
Seagrasses form important coastal ecosystems.[9] The worldwide endangering of these sea meadows, which provide food and habitat for many marine species, prompts the need for protection and understanding of these valuable resources.[10]
Evolution
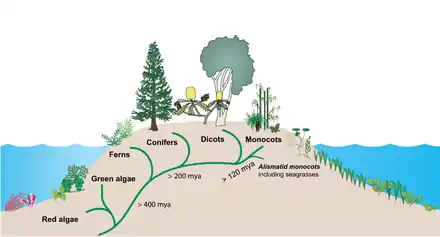
Showing the progression onto land from marine origins, the diversification of land plants and the subsequent return to the sea by the seagrasses
Around 140 million years ago, seagrasses evolved from early monocots which succeeded in conquering the marine environment.[10] Monocots are grass and grass-like flowering plants (angiosperms), the seeds of which typically contain only one embryonic leaf or cotyledon.[11]
Terrestrial plants evolved perhaps as early as 450 million years ago from a group of green algae.[12] Seagrasses then evolved from terrestrial plants which migrated back into the ocean.[13][14] Between about 70 million and 100 million years ago, three independent seagrass lineages (Hydrocharitaceae, Cymodoceaceae complex, and Zosteraceae) evolved from a single lineage of the monocotyledonous flowering plants.[15]
Other plants that colonised the sea, such as salt marsh plants, mangroves, and marine algae, have more diverse evolutionary lineages. In spite of their low species diversity, seagrasses have succeeded in colonising the continental shelves of all continents except Antarctica.[16]
Recent sequencing of the genomes of Zostera marina and Zostera muelleri has given a better understanding of angiosperm adaption to the sea.[17][18] During the evolutionary step back to the ocean, different genes have been lost (e.g., stomatal genes) or have been reduced (e.g., genes involved in the synthesis of terpenoids) and others have been regained, such as in genes involved in sulfation.[18][10]
Genome information has shown further that adaption to the marine habitat was accomplished by radical changes in cell wall composition.[17][18] However the cell walls of seagrasses are not well understood. In addition to the ancestral traits of land plants one would expect habitat-driven adaption process to the new environment characterized by multiple abiotic (high amounts of salt) and biotic (different seagrass grazers and bacterial colonization) stressors.[10] The cell walls of seagrasses seem intricate combinations of features known from both angiosperm land plants and marine macroalgae with new structural elements.[10]
Taxonomy
Today, seagrasses are a polyphyletic group of marine angiosperms with around 60 species in five families (Zosteraceae, Hydrocharitaceae, Posidoniaceae, Cymodoceaceae, and Ruppiaceae), which belong to the order Alismatales according to the Angiosperm Phylogeny Group IV System.[19] The genus Ruppia, which occurs in brackish water, is not regarded as a "real" seagrass by all authors and has been shifted to the Cymodoceaceae by some authors.[20] The APG IV system and The Plant List Webpage[21] do not share this family assignment.[10]
| Family | Image | Genera | Description |
|---|---|---|---|
| Zosteraceae | The family Zosteraceae, also known as the seagrass family, includes two genera containing 14 marine species. It is found in temperate and subtropical coastal waters, with the highest diversity located around Korea and Japan. Species subtotal: | ||
 |
Phyllospadix | 6 species | |
 |
Zostera | 16 species | |
| Hydrocharitaceae | The family Hydrocharitaceae, also known as tape-grasses, include Canadian waterweed and frogbit. The family includes both fresh and marine aquatics, although of the sixteen genera currently recognised, only three are marine.[22] They are found throughout the world in a wide variety of habitats, but are primarily tropical. Species subtotal: | ||
 |
Enhalus | 1 species | |
 |
Halophila | 19 species | |
 |
Thalassia | 2 species | |
| Posidoniaceae | The family Posidoniaceae contains a single genus with two to nine marine species found in the seas of the Mediterranean and around the south coast of Australia. Species subtotal: 2 to 9 | ||
.jpg.webp) |
Posidonia | 2 to 9 species | |
| Cymodoceaceae | The family Cymodoceaceae, also known as manatee-grass, includes only marine species.[23] Some taxonomists do not recognize this family. Species subtotal: | ||
 |
Amphibolis | 2 species | |
 |
Cymodocea | 4 species | |
 |
Halodule | 6 species | |
 |
Syringodium | 2 species | |
 |
Thalassodendron | 3 species | |
| Total species: | |||
Sexual recruitment
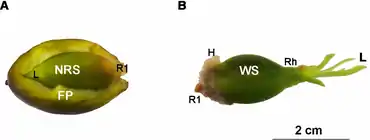
inside a fruit seeds

Seagrass populations are currently threatened by a variety of anthropogenic stressors.[25][7] The ability of seagrasses to cope with environmental perturbations depends, to some extent, on genetic variability, which is obtained through sexual recruitment.[26][27][28] By forming new individuals, seagrasses increase their genetic diversity and thus their ability to colonise new areas and to adapt to environmental changes.[29][30][31][32][33][24]
Seagrasses have contrasting colonisation strategies.[34] Some seagrasses form seed banks of small seeds with hard pericarps that can remain in the dormancy stage for several months. These seagrasses are generally short-lived and can recover quickly from disturbances by not germinating far away from parent meadows (e.g., Halophila sp., Halodule sp., Cymodocea sp., Zostera sp. and Heterozostera sp.[34][35] In contrast, other seagrasses form dispersal propagules. This strategy is typical of long-lived seagrasses that can form buoyant fruits with inner large non-dormant seeds, such as the genera Posidonia sp., Enhalus sp. and Thalassia sp.[34][36] Accordingly, the seeds of long-lived seagrasses have a large dispersal capacity compared to the seeds of the short-lived type,[37] which permits the evolution of species beyond unfavourable light conditions by the seedling development of parent meadows.[24]
The seagrass Posidonia oceanica (L.) Delile is one of the oldest and largest species on Earth. An individual can form meadows measuring nearly 15 km wide and can be hundreds to thousands of years old.[38] P. oceanica meadows play important roles in the maintenance of the geomorphology of Mediterranean coasts, which, among others, makes this seagrass a priority habitat of conservation.[39] Currently, the flowering and recruitment of P. oceanica seems to be more frequent than that expected in the past.[40][41][42][43][44] Further, this seagrass has singular adaptations to increase its survival during recruitment. The large amounts of nutrient reserves contained in the seeds of this seagrass support shoot and root growth, even up to the first year of seedling development.[38] In the first months of germination, when leaf development is scarce, P. oceanica seeds perform photosynthetic activity, which increases their photosynthetic rates and thus maximises seedling establishment success.[45][46] Seedlings also show high morphology plasticity during their root system development [47][48] by forming adhesive root hairs to help anchor themselves to rocky sediments.[40][49][50] However, many factors about P. oceanica sexual recruitment remain unknown, such as when photosynthesis in seeds is active or how seeds can remain anchored to and persist on substrate until their root systems have completely developed.[24]
Intertidal and subtidal

Seagrasses occurring in the intertidal and subtidal zones are exposed to highly variable environmental conditions due to tidal changes.[52][53] Subtidal seagrasses are more frequently exposed to lower light conditions, driven by plethora of natural and human-caused influences that reduce light penetration by increasing the density of suspended opaque materials. Subtidal light conditions can be estimated, with high accuracy, using artificial intelligence, enabling more rapid mitigation than was available using in situ techniques.[54] Seagrasses in the intertidal zone are regularly exposed to air and consequently experience extreme high and low temperatures, high photoinhibitory irradiance, and desiccation stress relative to subtidal seagrass.[53][55][56] Such extreme temperatures can lead to significant seagrass dieback when seagrasses are exposed to air during low tide.[57][58][59] Desiccation stress during low tide has been considered the primary factor limiting seagrass distribution at the upper intertidal zone.[60] Seagrasses residing the intertidal zone are usually smaller than those in the subtidal zone to minimize the effects of emergence stress.[61][58] Intertidal seagrasses also show light-dependent responses, such as decreased photosynthetic efficiency and increased photoprotection during periods of high irradiance and air exposure.[62][63]

In contrast, seagrasses in the subtidal zone adapt to reduced light conditions caused by light attenuation and scattering due to the overlaying water column and suspended particles.[65][66] Seagrasses in the deep subtidal zone generally have longer leaves and wider leaf blades than those in the shallow subtidal or intertidal zone, which allows more photosynthesis, in turn resulting in greater growth.[56] Seagrasses also respond to reduced light conditions by increasing chlorophyll content and decreasing the chlorophyll a/b ratio to enhance light absorption efficiency by using the abundant wavelengths efficiently.[67][68][69] As seagrasses in the intertidal and subtidal zones are under highly different light conditions, they exhibit distinctly different photoacclimatory responses to maximize photosynthetic activity and photoprotection from excess irradiance.
Seagrasses assimilate large amounts of inorganic carbon to achieve high level production.[70][71] Marine macrophytes, including seagrass, use both CO2 and HCO−
3 (bicarbonate) for photosynthetic carbon reduction.[72][73][74] Despite air exposure during low tide, seagrasses in the intertidal zone can continue to photosynthesize utilizing CO2 in the air.[75] Thus, the composition of inorganic carbon sources for seagrass photosynthesis probably varies between intertidal and subtidal plants. Because stable carbon isotope ratios of plant tissues change based on the inorganic carbon sources for photosynthesis,[76][77] seagrasses in the intertidal and subtidal zones may have different stable carbon isotope ratio ranges.
Seagrass meadows


Seagrass beds/meadows can be either monospecific (made up of a single species) or in mixed beds. In temperate areas, usually one or a few species dominate (like the eelgrass Zostera marina in the North Atlantic), whereas tropical beds usually are more diverse, with up to thirteen species recorded in the Philippines.
Seagrass beds are diverse and productive ecosystems, and can harbor hundreds of associated species from all phyla, for example juvenile and adult fish, epiphytic and free-living macroalgae and microalgae, mollusks, bristle worms, and nematodes. Few species were originally considered to feed directly on seagrass leaves (partly because of their low nutritional content), but scientific reviews and improved working methods have shown that seagrass herbivory is an important link in the food chain, feeding hundreds of species, including green turtles, dugongs, manatees, fish, geese, swans, sea urchins and crabs. Some fish species that visit/feed on seagrasses raise their young in adjacent mangroves or coral reefs.
Seagrasses trap sediment and slow down water movement, causing suspended sediment to settle out. Trapping sediment benefits coral by reducing sediment loads, improving photosynthesis for both coral and seagrass.[78]
Although often overlooked, seagrasses provide a number of ecosystem services.[79][80] Seagrasses are considered ecosystem engineers.[81][14][13] This means that the plants alter the ecosystem around them. This adjusting occurs in both physical and chemical forms. Many seagrass species produce an extensive underground network of roots and rhizome which stabilizes sediment and reduces coastal erosion.[82] This system also assists in oxygenating the sediment, providing a hospitable environment for sediment-dwelling organisms.[81] Seagrasses also enhance water quality by stabilizing heavy metals, pollutants, and excess nutrients.[83][14][13] The long blades of seagrasses slow the movement of water which reduces wave energy and offers further protection against coastal erosion and storm surge. Furthermore, because seagrasses are underwater plants, they produce significant amounts of oxygen which oxygenate the water column. These meadows account for more than 10% of the ocean's total carbon storage. Per hectare, it holds twice as much carbon dioxide as rain forests and can sequester about 27.4 million tons of CO2 annually.[84]
Seagrass meadows provide food for many marine herbivores. Sea turtles, manatees, parrotfish, surgeonfish, sea urchins and pinfish feed on seagrasses. Many other smaller animals feed on the epiphytes and invertebrates that live on and among seagrass blades.[85] Seagrass meadows also provide physical habitat in areas that would otherwise be bare of any vegetation. Due to this three dimensional structure in the water column, many species occupy seagrass habitats for shelter and foraging. It is estimated that 17 species of coral reef fish spend their entire juvenile life stage solely on seagrass flats.[86] These habitats also act as a nursery grounds for commercially and recreationally valued fishery species, including the gag grouper (Mycteroperca microlepis), red drum, common snook, and many others.[87][88] Some fish species utilize seagrass meadows and various stages of the life cycle. In a recent publication, Dr. Ross Boucek and colleagues discovered that two highly sought after flats fish, the common snook and spotted sea trout provide essential foraging habitat during reproduction.[89] Sexual reproduction is extremely energetically expensive to be completed with stored energy; therefore, they require seagrass meadows in close proximity to complete reproduction.[89] Furthermore, many commercially important invertebrates also reside in seagrass habitats including bay scallops (Argopecten irradians), horseshoe crabs, and shrimp. Charismatic fauna can also be seen visiting the seagrass habitats. These species include West Indian manatee, green sea turtles, and various species of sharks. The high diversity of marine organisms that can be found on seagrass habitats promotes them as a tourist attraction and a significant source of income for many coastal economies along the Gulf of Mexico and in the Caribbean.
 Thalassia testudinum seagrass bed
Thalassia testudinum seagrass bed White-spotted puffers, often found in seagrass areas
White-spotted puffers, often found in seagrass areas
Seagrass microbiome
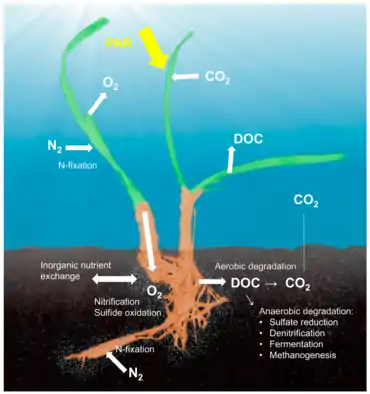
Seagrass holobiont
The concept of the holobiont, which emphasizes the importance and interactions of a microbial host with associated microorganisms and viruses and describes their functioning as a single biological unit,[92] has been investigated and discussed for many model systems, although there is substantial criticism of a concept that defines diverse host-microbe symbioses as a single biological unit.[93] The holobiont and hologenome concepts have evolved since the original definition,[94] and there is no doubt that symbiotic microorganisms are pivotal for the biology and ecology of the host by providing vitamins, energy and inorganic or organic nutrients, participating in defense mechanisms, or by driving the evolution of the host.[95] Although most work on host-microbe interactions has been focused on animal systems such as corals, sponges, or humans, there is a substantial body of literature on plant holobionts.[96] Plant-associated microbial communities impact both key components of the fitness of plants, growth and survival,[97] and are shaped by nutrient availability and plant defense mechanisms.[98] Several habitats have been described to harbor plant-associated microbes, including the rhizoplane (surface of root tissue), the rhizosphere (periphery of the roots), the endosphere (inside plant tissue), and the phyllosphere (total above-ground surface area).[90] The microbial community in the P. oceanica rhizosphere shows similar complexity as terrestrial habitats that contain thousands of taxa per gram of soil. In contrast, the chemistry in the rhizosphere of P. oceanica was dominated by the presence of sugars like sucrose and phenolics.[99]
Cell walls

Seagrass cell walls contain the same polysaccharides found in angiosperm land plants, such as cellulose[100] However, the cell walls of some seagrasses are characterised by sulfated polysaccharides,[101][102] which is a common attribute of macroalgae from the groups of red, brown and also green algae. It was proposed in 2005 that the ability to synthesise sulfated polysaccharides was regained by marine angiosperms.[101] Another unique feature of cell walls of seagrasses is the occurrence of unusual pectic polysaccharides called apiogalacturonans.[103][104][10]
In addition to polysaccharides, glycoproteins of the hydroxyproline-rich glycoprotein family,[105] are important components of cell walls of land plants. The highly glycosylated arabinogalactan proteins are of interest because of their involvement in both wall architecture and cellular regulatory processes.[106][107] Arabinogalactan proteins are ubiquitous in seed land plants [107] and have also been found in ferns, lycophytes and mosses.[108] They are structurally characterised by large polysaccharide moieties composed of arabinogalactans (normally over 90% of the molecule) which are covalently linked via hydroxyproline to relatively small protein/peptide backbones (normally less than 10% of the molecule).[107] Distinct glycan modifications have been identified in different species and tissues and it has been suggested these influence physical properties and function. In 2020, AGPs were isolated and structurally characterised for the first time from a seagrass.[109] Although the common backbone structure of land plant arabinogalactan proteins is conserved, the glycan structures exhibit unique features suggesting a role of seagrass arabinogalactan proteins in osmoregulation.[110][10]
Further components of secondary walls of plants are cross-linked phenolic polymers called lignin, which are responsible for mechanical strengthening of the wall. In seagrasses, this polymer has also been detected, but often in lower amounts compared to angiosperm land plants.[111][112][113][114][10] Thus, the cell walls of seagrasses seem to contain combinations of features known from both angiosperm land plants and marine macroalgae together with new structural elements. Dried seagrass leaves might be useful for papermaking or as insulating materials, so knowledge of cell wall composition has some technological relevance.[10]
Threats and conservation
Despite only covering 0.1 - 0.2% of the ocean’s surface, seagrasses form critically important ecosystems. Much like many other regions of the ocean, seagrasses have been faced with an accelerating global decline.[115] Since the late 19th century, over 20% of the global seagrass area has been lost, with seagrass bed loss occurring at a rate of 1.5% each year.[116] Of the 72 global seagrass species, approximately one quarter (15 species) could be considered at a Threatened or Near Threatened status on the IUCN’s Red List of Threatened Species.[117] Threats include a combination of natural factors, such as storms and disease, and anthropogenic in origin, including habitat destruction, pollution, and climate change.[115]
By far the most common threat to seagrass is human activity. Up to 67 species (93%) of seagrasses are affected by human activity along coastal regions.[117] Activities such as coastal land development, motorboating, and fishing practices like trawling either physically destroy seagrass beds or increase turbidity in the water, causing seagrass die-off. Since seagrasses have some of the highest light requirements of angiosperm plant species, they are highly affected by environmental conditions that change water clarity and block light.[118]
Seagrasses are also negatively affected by changing global climatic conditions. Increased weather events, sea level rise, and higher temperatures as a result of global warming all have the potential to induce widespread seagrass loss. An additional threat to seagrass beds is the introduction of non-native species. For seagrass beds worldwide, at least 28 non-native species have become established. Of these invasive species, the majority (64%) have been documented to infer negative effects on the ecosystem.[118]
Another major cause of seagrass disappearance is coastal eutrophication. Rapidly developing human population density along coastlines has led to high nutrient loads in coastal waters from sewage and other impacts of development. Increased nutrient loads create an accelerating cascade of direct and indirect effects that lead to seagrass decline. While some exposure to high concentrations of nutrients, especially nitrogen and phosphorus, can result in increased seagrass productivity, high nutrient levels can also stimulate the rapid overgrowth of macroalgae and epiphytes in shallow water, and phytoplankton in deeper water. In response to high nutrient levels, macroalgae form dense canopies on the surface of the water, limiting the light able to reach the benthic seagrasses.[119] Algal blooms caused by eutrophication also lead to hypoxic conditions, which seagrasses are also highly susceptible to. Since coastal sediment is generally anoxic, seagrass must supply oxygen to their below-ground roots either through photosynthesis or by the diffusion of oxygen in the water column. When the water surrounding seagrass becomes hypoxic, so too do seagrass tissues. Hypoxic conditions negatively affect seagrass growth and survival with seagrasses exposed to hypoxic conditions shown to have reduced rates of photosynthesis, increased respiration, and smaller growth. Hypoxic conditions can eventually lead to seagrass die-off which creates a positive feedback cycle, where the decomposition of organic matter further decreases the amount of oxygen present in the water column.[119]
Possible seagrass population trajectories have been studied in the Mediterranean sea. These studies suggest that the presence of seagrass depends on physical factors such as temperature, salinity, depth and turbidity, along with natural phenomena like climate change and anthropogenic pressure. While there are exceptions, regression was a general trend in many areas of the Mediterranean Sea. There is an estimated 27.7% reduction along the southern coast of Latium, 18%-38% reduction in the Northern Mediterranean basin, 19%-30% reduction on Ligurian coasts since the 1960s and 23% reduction in France in the past 50 years. In Spain the main reason for regression was due to human activity such as illegal trawling and aquaculture farming. It was found that areas with medium to high human impact suffered more severe reduction. Overall, it was suggested that 29% of known areal seagrass populations have disappeared since 1879. The reduction in these areas suggests that should warming in the Mediterranean basin continue, it may lead to a functional extinction of Posidonia oceanica in the Mediterranean by 2050. Scientists suggested that the trends they identified appear to be part of a large-scale trend worldwide.[120]
Conservation efforts are imperative to the survival of seagrass species. While there are many challenges to overcome with respect to seagrass conservation there are some major ones that can be addressed. Societal awareness of what seagrasses are and their importance to human well-being is incredibly important. As the majority of people become more urbanized they are increasingly more disconnected from the natural world. This allows for misconceptions and a lack of understanding of seagrass ecology and its importance. Additionally, it is a challenge to obtain and maintain information on the status and condition of seagrass populations. With many populations across the globe, it is difficult to map the current populations. Another challenge faced in seagrass conservation is the ability to identify threatening activities on a local scale. Also, in an ever growing human population, there is a need to balance the needs of the people while also balancing the needs of the planet. Lastly, it is challenging to generate scientific research to support conservation of seagrass. Limited efforts and resources are dedicated to the study of seagrasses.[121] This is seen in areas such as India and China where there is little to no plan in place to conserve seagrass populations. However, the conservation and restoration of seagrass may contribute to 16 of the 17 UN Sustainable Development Goals.[122]
In a study of seagrass conservation in China, several suggestions were made by scientists on how to better conserve seagrass. They suggested that seagrass beds should be included in the Chinese conservation agenda as done in other countries. They called for the Chinese government to forbid land reclamation in areas near or in seagrass beds, to reduce the number and size of culture ponds, to control raft aquaculture and improve sediment quality, to establish seagrass reserves, to increase awareness of seagrass beds to fishermen and policy makers and to carry out seagrass restoration.[123] Similar suggestions were made in India where scientists suggested that public engagement was important. Also, scientists, the public, and government officials should work in tandem to integrate traditional ecological knowledge and socio-cultural practices to evolve conservation policies.[124]
World Seagrass Day is an annual event held on March 1 to raise awareness about seagrass and its important functions in the marine ecosystem.[125][126]
See also
- Alismatales
- Blue carbon
- Salt marsh
- Mangrove
- Sea rewilding
- Nursery habitat
- Ocean Data Viewer: contains the global distribution of seagrasses dataset
References
- Tomlinson and Vargo (1966). "On the morphology and anatomy of turtle grass, Thalassia testudinum (Hydrocharitaceae). I. Vegetative Morphology". Bulletin of Marine Science. 16: 748–761.
- van Tussenbroek, Brigitta I.; Villamil, Nora; Márquez-Guzmán, Judith; Wong, Ricardo; Monroy-Velázquez, L. Verónica; Solis-Weiss, Vivianne (29 September 2016). "Experimental evidence of pollination in marine flowers by invertebrate fauna". Nature Communications. 7 (1): 12980. Bibcode:2016NatCo...712980V. doi:10.1038/ncomms12980. ISSN 2041-1723. PMC 5056424. PMID 27680661.
- "39 Ways to Save the Planet - Sublime Seagrass". BBC Radio 4. BBC. Retrieved 12 February 2022.
- Papenbrock, Jutta (2012). "Highlights in Seagrasses' Phylogeny, Physiology, and Metabolism: What Makes Them Special?". ISRN Botany. 2012: 1–15. doi:10.5402/2012/103892.
 Material was copied from this source, which is available under a Creative Commons Attribution 3.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 3.0 International License. - Larkum A. W. D., R. J. Orth, and C. M. Duarte (2006) Seagrass: Biology, Ecology and Conservation, Springer, The Netherlands.
- Orth, Robert J.; Carruthers, TIM J. B.; Dennison, William C.; Duarte, Carlos M.; Fourqurean, James W.; Heck, Kenneth L.; Hughes, A. Randall; Kendrick, Gary A.; Kenworthy, W. Judson; Olyarnik, Suzanne; Short, Frederick T.; Waycott, Michelle; Williams, Susan L. (2006). "A Global Crisis for Seagrass Ecosystems". BioScience. 56 (12): 987. doi:10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2. ISSN 0006-3568. S2CID 4936412.
- Waycott, M.; Duarte, C. M.; Carruthers, T. J. B.; Orth, R. J.; Dennison, W. C.; Olyarnik, S.; Calladine, A.; Fourqurean, J. W.; Heck, K. L.; Hughes, A. R.; Kendrick, G. A.; Kenworthy, W. J.; Short, F. T.; Williams, S. L. (2009). "Accelerating loss of seagrasses across the globe threatens coastal ecosystems". Proceedings of the National Academy of Sciences. 106 (30): 12377–12381. Bibcode:2009PNAS..10612377W. doi:10.1073/pnas.0905620106. PMC 2707273. PMID 19587236.
- Short, Frederick T.; Polidoro, Beth; Livingstone, Suzanne R.; Carpenter, Kent E.; Bandeira, Salomão; Bujang, Japar Sidik; Calumpong, Hilconida P.; Carruthers, Tim J.B.; Coles, Robert G.; Dennison, William C.; Erftemeijer, Paul L.A.; Fortes, Miguel D.; Freeman, Aaren S.; Jagtap, T.G.; Kamal, Abu Hena M.; Kendrick, Gary A.; Judson Kenworthy, W.; La Nafie, Yayu A.; Nasution, Ichwan M.; Orth, Robert J.; Prathep, Anchana; Sanciangco, Jonnell C.; Tussenbroek, Brigitta van; Vergara, Sheila G.; Waycott, Michelle; Zieman, Joseph C. (2011). "Extinction risk assessment of the world's seagrass species" (PDF). Biological Conservation. 144 (7): 1961–1971. doi:10.1016/j.biocon.2011.04.010. S2CID 32533417.
- Hemminga, M. A., and Duarte, C. M. eds (2000). “Seagrasses in the human environment,” in Seagrass Ecology (Cambridge: Cambridge University Press), 248–291.
- Pfeifer, Lukas; Classen, Birgit (2020). "The Cell Wall of Seagrasses: Fascinating, Peculiar and a Blank Canvas for Future Research". Frontiers in Plant Science. 11: 588754. doi:10.3389/fpls.2020.588754. PMC 7644952. PMID 33193541.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Rudall, Paula J.; Buzgo, Matyas (2002). "Evolutionary history of the monocot leaf". Developmental Genetics and Plant Evolution. Systematics Association Special Volumes. Vol. 20020544. pp. 431–458. doi:10.1201/9781420024982.ch23. ISBN 978-0-415-25790-9., in Cronk, Bateman & Hawkins (2002)
- Knauth, L. Paul; Kennedy, Martin J. (2009). "The late Precambrian greening of the Earth". Nature. 460 (7256): 728–732. Bibcode:2009Natur.460..728K. doi:10.1038/nature08213. PMID 19587681. S2CID 4398942.
- Orth; et al. (2006). "A global crisis for seagrass ecosystems". BioScience. 56 (12): 987–996. doi:10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2. hdl:10261/88476. S2CID 4936412.
- Papenbrock, J (2012). "Highlights in seagrass' phylogeny, physiology, and metabolism: what makes them so species?". International Scholarly Research Network: 1–15.
- Les, D.H., Cleland, M.A. and Waycott, M. (1997) "Phylogenetic studies in Alismatidae, II: evolution of marine angiosperms (seagrasses) and hydrophily". Systematic Botany 22(3): 443–463.
- Orth, Robert J.; Carruthers, TIM J. B.; Dennison, William C.; Duarte, Carlos M.; Fourqurean, James W.; Heck, Kenneth L.; Hughes, A. Randall; Kendrick, Gary A.; Kenworthy, W. Judson; Olyarnik, Suzanne; Short, Frederick T.; Waycott, Michelle; Williams, Susan L. (2006). "A Global Crisis for Seagrass Ecosystems". BioScience. 56 (12): 987. doi:10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2. ISSN 0006-3568. S2CID 4936412.
- Lee, Hueytyng; Golicz, Agnieszka A.; Bayer, Philipp E.; Jiao, Yuannian; Tang, Haibao; Paterson, Andrew H.; Sablok, Gaurav; Krishnaraj, Rahul R.; Chan, Chon-Kit Kenneth; Batley, Jacqueline; Kendrick, Gary A.; Larkum, Anthony W.D.; Ralph, Peter J.; Edwards, David (2016). "The Genome of a Southern Hemisphere Seagrass Species (Zostera muelleri)". Plant Physiology. 172 (1): 272–283. doi:10.1104/pp.16.00868. PMC 5074622. PMID 27373688.
- Olsen, Jeanine L.; et al. (2016). "The genome of the seagrass Zostera marina reveals angiosperm adaptation to the sea". Nature. 530 (7590): 331–335. Bibcode:2016Natur.530..331O. doi:10.1038/nature16548. PMID 26814964. S2CID 3713147.
- "An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV". Botanical Journal of the Linnean Society. 181: 1–20. 2016. doi:10.1111/boj.12385. S2CID 7498637.
- Wilkin, Paul; Mayo, Simon J. (30 May 2013). Early Events in Monocot Evolution. ISBN 9781107244603.
- The Plant List (2020). Ruppia. Available online at: http://www.theplantlist.org/1.1/browse/A/Ruppiaceae/Ruppia/ (accessed September 22, 2020).
- Christenhusz, Maarten J.M.; Byng, James W. (20 May 2016). "The number of known plants species in the world and its annual increase". Phytotaxa. 261 (3): 201. doi:10.11646/phytotaxa.261.3.1. ISSN 1179-3163.
- Waycott, Michelle; McMahon, Kathryn; Lavery, Paul (2014). A Guide to Southern Temperate Seagrasses. CSIRO Publishing. ISBN 9781486300150.
- Guerrero-Meseguer, Laura; Sanz-Lázaro, Carlos; Marín, Arnaldo (2018). "Understanding the sexual recruitment of one of the oldest and largest organisms on Earth, the seagrass Posidonia oceanica". PLOS ONE. 13 (11): e0207345. Bibcode:2018PLoSO..1307345G. doi:10.1371/journal.pone.0207345. PMC 6239318. PMID 30444902.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Collier, C.J.; Waycott, M. (2014). "Temperature extremes reduce seagrass growth and induce mortality". Marine Pollution Bulletin. 83 (2): 483–490. doi:10.1016/j.marpolbul.2014.03.050. PMID 24793782.
- Reusch, T. B. H.; Ehlers, A.; Hammerli, A.; Worm, B. (2005). "Ecosystem recovery after climatic extremes enhanced by genotypic diversity". Proceedings of the National Academy of Sciences. 102 (8): 2826–2831. Bibcode:2005PNAS..102.2826R. doi:10.1073/pnas.0500008102. PMC 549506. PMID 15710890.
- Guerrero-Meseguer, Laura; Sanz-Lázaro, Carlos; Marín, Arnaldo (2018). "Understanding the sexual recruitment of one of the oldest and largest organisms on Earth, the seagrass Posidonia oceanica". PLOS ONE. 13 (11): e0207345. Bibcode:2018PLoSO..1307345G. doi:10.1371/journal.pone.0207345. PMC 6239318. PMID 30444902.
- Ehlers, A.; Worm, B.; Reusch, TBH (2008). "Importance of genetic diversity in eelgrass Zostera marina for its resilience to global warming". Marine Ecology Progress Series. 355: 1–7. Bibcode:2008MEPS..355....1E. doi:10.3354/meps07369.
- Orth, Robert J.; Harwell, Matthew C.; Inglis, Graeme J. (2006). "Ecology of Seagrass Seeds and Seagrass Dispersal Processes". Seagrasses: Biology, Ecologyand Conservation. pp. 111–133. doi:10.1007/978-1-4020-2983-7_5. ISBN 978-1-4020-2942-4.
- Van Dijk, JK; Van Tussenbroek, BI; Jiménez-Durán, K.; Márquez-Guzmán, GJ; Ouborg, J. (2009). "High levels of gene flow and low population genetic structure related to high dispersal potential of a tropical marine angiosperm". Marine Ecology Progress Series. 390: 67–77. Bibcode:2009MEPS..390...67V. doi:10.3354/meps08190.
- Intergovernmental Panel On Climate Change, ed. (2014). "Summary for Policymakers". Climate Change 2013 - the Physical Science Basis. pp. 1–30. doi:10.1017/CBO9781107415324.004. hdl:10818/26263. ISBN 9781107415324.
- McMahon, Kathryn; Van Dijk, Kor-Jent; Ruiz-Montoya, Leonardo; Kendrick, Gary A.; Krauss, Siegfried L.; Waycott, Michelle; Verduin, Jennifer; Lowe, Ryan; Statton, John; Brown, Eloise; Duarte, Carlos (2014). "The movement ecology of seagrasses". Proceedings of the Royal Society B: Biological Sciences. 281 (1795). doi:10.1098/rspb.2014.0878. PMC 4213608. PMID 25297859.
- Smith, Timothy M.; York, Paul H.; MacReadie, Peter I.; Keough, Michael J.; Ross, D. Jeff; Sherman, Craig D.H. (2016). "Spatial variation in reproductive effort of a southern Australian seagrass". Marine Environmental Research. 120: 214–224. doi:10.1016/j.marenvres.2016.08.010. PMID 27592387.
- j. Inglis, Graeme (1999). "Variation in the recruitment behaviour of seagrass seeds: Implications for population dynamics and resource management". Pacific Conservation Biology. 5 (4): 251. doi:10.1071/PC000251.
- Kuo, John; Iizumi, Hitoshi; Nilsen, Bjorg E.; Aioi, Keiko (1990). "Fruit anatomy, seed germination and seedling development in the Japanese seagrass Phyllospadix (Zosteraceae)". Aquatic Botany. 37 (3): 229–245. doi:10.1016/0304-3770(90)90072-S.
- Kuo J, Den Hartog C. (2006) "Seagrass morphology, anatomy, and ultrastructure". In: Larkum AWD, Orth RJ, Duarte CM (Eds), Seagrasses: Biology, Ecology and Conservation, Springer, pages 51–87.
- Fonseca, Mark S.; Kenworthy, W.Judson (1987). "Effects of current on photosynthesis and distribution of seagrasses". Aquatic Botany. 27: 59–78. doi:10.1016/0304-3770(87)90086-6.
- Arnaud-Haond, Sophie; Duarte, Carlos M.; Diaz-Almela, Elena; Marbà, Núria; Sintes, Tomas; Serrão, Ester A. (2012). "Implications of Extreme Life Span in Clonal Organisms: Millenary Clones in Meadows of the Threatened Seagrass Posidonia oceanica". PLOS ONE. 7 (2): e30454. Bibcode:2012PLoSO...730454A. doi:10.1371/journal.pone.0030454. PMC 3270012. PMID 22312426.
- Vacchi, Matteo; De Falco, Giovanni; Simeone, Simone; Montefalcone, Monica; Morri, Carla; Ferrari, Marco; Bianchi, Carlo Nike (2017). "Biogeomorphology of the Mediterranean Posidonia oceanicaseagrass meadows". Earth Surface Processes and Landforms. 42 (1): 42–54. Bibcode:2017ESPL...42...42V. doi:10.1002/esp.3932. S2CID 130872337.
- Tripathi, Durgesh K.; Mishra, Rohit K.; Singh, Swati; Singh, Samiksha; Vishwakarma, Kanchan; Sharma, Shivesh; Singh, Vijay P.; Singh, Prashant K.; Prasad, Sheo M.; Dubey, Nawal K.; Pandey, Avinash C.; Sahi, Shivendra; Chauhan, Devendra K. (2017). "Nitric Oxide Ameliorates Zinc Oxide Nanoparticles Phytotoxicity in Wheat Seedlings: Implication of the Ascorbate–Glutathione Cycle". Frontiers in Plant Science. 8: 1. doi:10.3389/fpls.2017.00001. PMC 5292406. PMID 28220127.
- Montefalcone, M.; Giovannetti, E.; Morri, C.; Peirano, A.; Bianchi, C. N. (2013). "Flowering of the seagrass Posidonia oceanica in NW Mediterranean: Is there a link with solar activity?". Mediterranean Marine Science. 14 (2): 416. doi:10.12681/mms.529. S2CID 85362624.
- Ruiz, J.M.; Marín-Guirao, L.; García-Muñoz, R.; Ramos-Segura, A.; Bernardeau-Esteller, J.; Pérez, M.; Sanmartí, N.; Ontoria, Y.; Romero, J.; Arthur, R.; Alcoverro, T.; Procaccini, G. (2018). "Experimental evidence of warming-induced flowering in the Mediterranean seagrass Posidonia oceanica". Marine Pollution Bulletin. 134: 49–54. doi:10.1016/j.marpolbul.2017.10.037. PMID 29102072. S2CID 11523686.
- Diaz-Almela, Elena; Marbà, Nuria; Duarte, Carlos M. (2007). "Consequences of Mediterranean warming events in seagrass (Posidonia oceanica) flowering records". Global Change Biology. 13 (1): 224–235. Bibcode:2007GCBio..13..224D. doi:10.1111/j.1365-2486.2006.01260.x. S2CID 84055440.
- Balestri, E.; Gobert, S.; Lepoint, G.; Lardicci, C. (2009). "Seed nutrient content and nutritional status of Posidonia oceanica seedlings in the northwestern Mediterranean Sea". Marine Ecology Progress Series. 388: 99–109. Bibcode:2009MEPS..388...99B. doi:10.3354/meps08104.
- Celdrán, David; Marín, Arnaldo (2011). "Photosynthetic activity of the non-dormant Posidonia oceanica seed". Marine Biology. 158 (4): 853–858. doi:10.1007/s00227-010-1612-4. S2CID 84357626.
- Celdrán, David; Marín, Arnaldo (2013). "Seed photosynthesis enhances Posidonia oceanicaseedling growth". Ecosphere. 4 (12): art149. doi:10.1890/ES13-00104.1.
- Balestri, Elena; De Battisti, Davide; Vallerini, Flavia; Lardicci, Claudio (2015). "First evidence of root morphological and architectural variations in young Posidonia oceanica plants colonizing different substrate typologies". Estuarine, Coastal and Shelf Science. 154: 205–213. Bibcode:2015ECSS..154..205B. doi:10.1016/j.ecss.2015.01.002. hdl:11568/755031.
- Guerrero-Meseguer, Laura; Sanz-Lázaro, Carlos; Suk-Ueng, Krittawit; Marín, Arnaldo (2017). "Influence of substrate and burial on the development of Posidonia oceanica : Implications for restoration". Restoration Ecology. 25 (3): 453–458. doi:10.1111/rec.12438. hdl:10045/66474. S2CID 88876962.
- Badalamenti, Fabio; Alagna, Adriana; Fici, Silvio (2015). "Evidences of adaptive traits to rocky substrates undermine paradigm of habitat preference of the Mediterranean seagrass Posidonia oceanica". Scientific Reports. 5: 8804. Bibcode:2015NatSR...5E8804B. doi:10.1038/srep08804. PMC 4350093. PMID 25740176.
- Alagna, Adriana; Fernández, Tomás Vega; Terlizzi, Antonio; Badalamenti, Fabio (2013). "Influence of microhabitat on seedling survival and growth of the mediterranean seagrass posidonia oceanica (L.) Delile". Estuarine, Coastal and Shelf Science. 119: 119–125. Bibcode:2013ECSS..119..119A. doi:10.1016/j.ecss.2013.01.009.
- Park, Sang Rul; Kim, Sangil; Kim, Young Kyun; Kang, Chang-Keun; Lee, Kun-Seop (2016). "Photoacclimatory Responses of Zostera marina in the Intertidal and Subtidal Zones". PLOS ONE. 11 (5): e0156214. Bibcode:2016PLoSO..1156214P. doi:10.1371/journal.pone.0156214. PMC 4881947. PMID 27227327.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Silva, J.; Santos, R. (2003). "Daily variation patterns in seagrass photosynthesis along a vertical gradient". Marine Ecology Progress Series. 257: 37–44. Bibcode:2003MEPS..257...37S. doi:10.3354/meps257037.
- Boese, Bruce L.; Robbins, Bradley D.; Thursby, Glen (2005). "Desiccation is a limiting factor for eelgrass (Zostera marina L.) distribution in the intertidal zone of a northeastern Pacific (USA) estuary". Botanica Marina. 48 (4). doi:10.1515/BOT.2005.037. S2CID 85105171.
- Pearson, Ryan M.; Collier, Catherine J.; Brown, Christopher J.; Rasheed, Michael A.; Bourner, Jessica; Turschwell, Mischa P.; Sievers, Michael; Connolly, Rod M. (15 August 2021). "Remote estimation of aquatic light environments using machine learning: A new management tool for submerged aquatic vegetation". Science of the Total Environment. 782: 146886. Bibcode:2021ScTEn.782n6886P. doi:10.1016/j.scitotenv.2021.146886. ISSN 0048-9697. S2CID 233519731.
- Durako, M. J.; Kunzelman, J. I.; Kenworthy, W. J.; Hammerstrom, K. K. (2003). "Depth-related variability in the photobiology of two populations of Halophila johnsonii and Halophila decipiens". Marine Biology. 142 (6): 1219–1228. doi:10.1007/s00227-003-1038-3. S2CID 85627116.
- Olivé, I.; Vergara, J. J.; Pérez-Lloréns, J. L. (2013). "Photosynthetic and morphological photoacclimation of the seagrass Cymodocea nodosa to season, depth and leaf position". Marine Biology. 160 (2): 285–297. doi:10.1007/s00227-012-2087-2. S2CID 86386210.
- Hemminga M. A. and Durate C. M. (2000) Seagrass ecology. Cambridge University Press.
- Seddon, S.; Cheshire, AC (2001). "Photosynthetic response of Amphibolis antarctica and Posidonia australis to temperature and desiccation using chlorophyll fluorescence". Marine Ecology Progress Series. 220: 119–130. Bibcode:2001MEPS..220..119S. doi:10.3354/meps220119.
- Hirst A, Ball D, Heislers S, Young P, Blake S, Coots A. Baywide Seagrass Monitoring Program, Milestone Report No. 2 (2008). Fisheries Victoria Technical Report No. 29, January 2009.
- Koch, Evamaria W. (2001). "Beyond Light: Physical, Geological, and Geochemical Parameters as Possible Submersed Aquatic Vegetation Habitat Requirements". Estuaries. 24 (1): 1–17. doi:10.2307/1352808. JSTOR 1352808. S2CID 85287808.
- Tanaka, Y.; Nakaoka, M. (2004). "Emergence stress and morphological constraints affect the species distribution and growth of subtropical intertidal seagrasses". Marine Ecology Progress Series. 284: 117–131. Bibcode:2004MEPS..284..117T. doi:10.3354/meps284117.
- Björk, M.; Uku, J.; Weil, A.; Beer, S. (1999). "Photosynthetic tolerances to desiccation of tropical intertidal seagrasses". Marine Ecology Progress Series. 191: 121–126. Bibcode:1999MEPS..191..121B. doi:10.3354/meps191121.
- Petrou, K.; Jimenez-Denness, I.; Chartrand, K.; McCormack, C.; Rasheed, M.; Ralph, PJ (2013). "Seasonal heterogeneity in the photophysiological response to air exposure in two tropical intertidal seagrass species" (PDF). Marine Ecology Progress Series. 482: 93–106. Bibcode:2013MEPS..482...93P. doi:10.3354/meps10229. hdl:10453/23914.
- Xu, Shaochun; Zhou, Yi; Wang, Pengmei; Wang, Feng; Zhang, Xiaomei; Gu, Ruiting (2016). "Salinity and temperature significantly influence seed germination, seedling establishment, and seedling growth of eelgrass Zostera marinaL". PeerJ. 4: e2697. doi:10.7717/peerj.2697. PMC 5119234. PMID 27896031.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Schwarz, A.-M.; Björk, M.; Buluda, T.; Mtolera, M.; Beer, S. (2000). "Photosynthetic utilisation of carbon and light by two tropical seagrass species as measured in situ". Marine Biology. 137 (5–6): 755–761. doi:10.1007/s002270000433. S2CID 86384408.
- Campbell, Stuart J.; McKenzie, Len J.; Kerville, Simon P.; Bité, Juanita S. (2007). "Patterns in tropical seagrass photosynthesis in relation to light, depth and habitat". Estuarine, Coastal and Shelf Science. 73 (3–4): 551–562. Bibcode:2007ECSS...73..551C. doi:10.1016/j.ecss.2007.02.014.
- Lee, Kun-Seop; Dunton, Kenneth H. (1997). "Effect of in situ light reduction on the maintenance, growth and partitioning of carbon resources in Thalassia testudinum banks ex König". Journal of Experimental Marine Biology and Ecology. 210: 53–73. doi:10.1016/S0022-0981(96)02720-7.
- Longstaff, B.J; Dennison, W.C (1999). "Seagrass survival during pulsed turbidity events: The effects of light deprivation on the seagrasses Halodule pinifolia and Halophila ovalis". Aquatic Botany. 65 (1–4): 105–121. doi:10.1016/S0304-3770(99)00035-2.
- Collier, CJ; Lavery, PS; Ralph, PJ; Masini, RJ (2008). "Physiological characteristics of the seagrass Posidonia sinuosa along a depth-related gradient of light availability". Marine Ecology Progress Series. 353: 65–79. Bibcode:2008MEPS..353...65C. doi:10.3354/meps07171.
- Lee, Kun-Seop; Park, Sang Rul; Kim, Young Kyun (2007). "Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: A review". Journal of Experimental Marine Biology and Ecology. 350 (1–2): 144–175. doi:10.1016/j.jembe.2007.06.016.
- Nayar, S.; Collings, G.J.; Miller, D.J.; Bryars, S.; Cheshire, A.C. (2009). "Uptake and resource allocation of inorganic carbon by the temperate seagrasses Posidonia and Amphibolis". Journal of Experimental Marine Biology and Ecology. 373 (2): 87–95. doi:10.1016/j.jembe.2009.03.010.
- Beer, Sven (1989). "Photosynthesis and photorespiration of marine angiosperms". Aquatic Botany. 34 (1–3): 153–166. doi:10.1016/0304-3770(89)90054-5.
- Larkum AWD, James PL. Towards a model for inorganic carbon uptake in seagrasses involving carbonic anhydrase. In Kuo J, Phillips RC, Walker DI, Kirkman H, editors. Seagrass biology: Proceedings of an International Workshop. Nedlands: The University of Western Australia; 1996. pp. 191–196.
- Beer, Sven; Rehnberg, Jon (1997). "The acquisition of inorganic carbon by the seagrass Zostera marina". Aquatic Botany. 56 (3–4): 277–283. doi:10.1016/S0304-3770(96)01109-6.
- Silva, João; Santos, Rui; Calleja, Maria Ll.; Duarte, Carlos M. (2005). "Submerged versus air-exposed intertidal macrophyte productivity: From physiological to community-level assessments". Journal of Experimental Marine Biology and Ecology. 317: 87–95. doi:10.1016/j.jembe.2004.11.010.
- O'Leary, Marion H. (1988). "Carbon Isotopes in Photosynthesis". BioScience. 38 (5): 328–336. doi:10.2307/1310735. JSTOR 1310735.
- Raven, John A.; Johnston, Andrew M.; Kübler, Janet E.; Korb, Rebecca; McInroy, Shona G.; Handley, Linda L.; Scrimgeour, Charlie M.; Walker, Diana I.; Beardall, John; Vanderklift, Mathew; Fredriksen, Stein; Dunton, Kenneth H. (2002). "Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses". Functional Plant Biology. 29 (3): 355–378. doi:10.1071/PP01201. PMID 32689482.
- Seagrass-Watch: What is seagrass? Retrieved 2012-11-16.
- Nordlund, Lina; Koch, Evamaria W.; Barbier, Edward B.; Creed, Joel C. (12 October 2016). Reinhart, Kurt O. (ed.). "Seagrass Ecosystem Services and Their Variability across Genera and Geographical Regions". PLOS ONE. 11 (10): e0163091. Bibcode:2016PLoSO..1163091M. doi:10.1371/journal.pone.0163091. ISSN 1932-6203. PMC 5061329. PMID 27732600.
- United Nations Environment Programme (2020). Out of the blue: The value of seagrasses to the environment and to people. UNEP, Nairobi. https://www.unenvironment.org/resources/report/out-blue-value-seagrasses-environment-and-people
- Jones, Clive G.; Lawton, John H.; Shachak, Moshe (1994). "Organisms as ecosystem engineers". Oikos. 69 (3): 373–386. doi:10.2307/3545850. JSTOR 3545850.
- Grey, William; Moffler, Mark (1987). "Flowering of the seagrass Thalassia testudinum (Hydrocharitacea) in the Tampa Bay, Florida area". Aquatic Botany. 5: 251–259. doi:10.1016/0304-3770(78)90068-2.
- Darnell, Kelly; Dunton, Kenneth (2016). "Reproductive phenology of the subtropical seagrasses Thalassia testudinum (Turtle grass) and Halodule wrightii (Shoal grass) in the northwest Gulf of Mexico". Botanica Marina. 59 (6): 473–483. doi:10.1515/bot-2016-0080. S2CID 88685282.
- Macreadie, P. I.; Baird, M. E.; Trevathan-Tackett, S. M.; Larkum, A. W. D.; Ralph, P. J. (2013). "Quantifying and modelling the carbon sequestration capacity of seagrass meadows". Marine Pollution Bulletin. 83 (2): 430–439. doi:10.1016/j.marpolbul.2013.07.038. PMID 23948090.
- "Seagrass FAQ".
- Nagelkerken, I.; Roberts, C. M.; van der Velde, G.; Dorenbosch, M.; van Riel, M. C.; Cocheret de la Morinière, E.; Nienhuis, P. H. (2002). "How important are mangroves and seagrass beds for coral-reef fish? The nursery hypothesis tested on an island scale". Marine Ecology Progress Series. 244: 299–305. Bibcode:2002MEPS..244..299N. doi:10.3354/meps244299.
- Nordlund, L. M.; Unsworth, R. K. F.; Gullstrom, M.; Cullen-Unsworth, L. C. (2018). "Global significance of seagrass fishery activity". Fish and Fisheries. 19 (3): 399–412. doi:10.1111/faf.12259.
- Unsworth, R. K. F.; Nordlund, L. M.; Cullen-Unsworth, L. C. (2019). "Seagrass meadows support global fisheries production". Conserv Lett. e12566: e12566. doi:10.1111/conl.12566.
- Boucek, R. E.; Leone, E.; Bickford, J.; Walters-Burnsed, S.; Lowerre-Barbieri, S. (2017). "More than just a spawning location: Examining fine-scale s[ace use of two estuarine fish species at a spawning aggregation site". Frontiers in Marine Science (4): 1–9.
- Ugarelli, K., Chakrabarti, S., Laas, P. and Stingl, U. (2017) "The seagrass holobiont and its microbiome". Microorganisms, 5(4): 81. doi:10.3390/microorganisms5040081.
 Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License.
Material was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. - Tarquinio, F., Hyndes, G.A., Laverock, B., Koenders, A. and Säwström, C. (2019) "The seagrass holobiont: understanding seagrass-bacteria interactions and their role in seagrass ecosystem functioning". FEMS microbiology letters, 366(6): fnz057. doi:10.1093/femsle/fnz057.
- Margulis, Lynn (1991) "Symbiogenesis and Symbionticism". In: Symbiosis as a Source of Evolutionary Innovation; Margulis, L., Fester, R.(Eds.), Cambridge MIT Press. ISBN 9780262132695.
- Douglas, A.E.; Werren, J.H. (2016) "Holes in the Hologenome: Why Host-Microbe Symbioses Are Not Holobionts". mBio, 7: e02099-15. doi:10.1128/mBio.02099-15.
- Theis, K.R.; Dheilly, N.M.; Klassen, J.L.; Brucker, R.M.; Baines, J.F.; Bosch, T.C.G.; Cryan, J.F.; Gilbert, S.F.; Goodnight, C.J.; Lloyd, E.A.; et al. Getting the Hologenome Concept Right: An Eco-Evolutionary Framework for Hosts and Their Microbiomes. mSystems 2016, 1, e00028-16. doi:10.1128/mSystems.00028-16.
- Rosenberg, E. and Zilber-Rosenberg, I. (2016) "Microbes drive evolution of animals and plants: the hologenome concept". MBio, 7(2). doi:10.1128/mBio.01395-15.
- Zilber-Rosenberg, I. and Rosenberg, E. (2008) "Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution". FEMS Microbiology Reviews, 32(5): 723–735. doi:10.1111/j.1574-6976.2008.00123.x.
- Vandenkoornhuyse, P., Quaiser, A., Duhamel, M., Le Van, A. and Dufresne, A. (2015) "The importance of the microbiome of the plant holobiont". New Phytologist, 206(4): 1196-1206. doi:10.1111/nph.13312.
- Sánchez-Cañizares, C., Jorrín, B., Poole, P.S. and Tkacz, A. (2017) "Understanding the holobiont: the interdependence of plants and their microbiome". Current Opinion in Microbiology, 38: 188–196. doi:10.1016/j.mib.2017.07.001.
- Sogin, E. Maggie; Michellod, Dolma; Gruber-Vodicka, Harald R.; Bourceau, Patric; Geier, Benedikt; Meier, Dimitri V.; Seidel, Michael; Ahmerkamp, Soeren; Schorn, Sina; D’Angelo, Grace; Procaccini, Gabriele (2 May 2022). "Sugars dominate the seagrass rhizosphere". Nature Ecology & Evolution. 6 (7): 866–877. doi:10.1038/s41559-022-01740-z. ISSN 2397-334X. PMC 9262712. PMID 35501482.
- Syed, Nurul Farahin Nur; Zakaria, Muta Harah; Bujang, Japar Sidik (2016). "Fiber Characteristics and Papermaking of Seagrass Using Hand-beaten and Blended Pulp". BioResources. 11 (2). doi:10.15376/biores.11.2.5358-5380.
- Aquino, R. S.; Landeira-Fernandez, A. M.; Valente, A. P.; Andrade, L. R.; Mourão, P. A. (2004). "Occurrence of sulfated galactans in marine angiosperms: Evolutionary implications". Glycobiology. 15 (1): 11–20. doi:10.1093/glycob/cwh138. PMID 15317737.
- Silva, Juliana M. C.; Dantas-Santos, Nednaldo; Gomes, Dayanne L.; Costa, Leandro S.; Cordeiro, Sara L.; Costa, Mariana S. S. P.; Silva, Naisandra B.; Freitas, Maria L.; Scortecci, Katia Castanho; Leite, Edda L.; Rocha, Hugo A. O. (2012). "Biological activities of the sulfated polysaccharide from the vascular plant Halodule wrightii". Revista Brasileira de Farmacognosia. 22: 94–101. doi:10.1590/S0102-695X2011005000199.
- Gloaguen, Vincent; Brudieux, Véronique; Closs, Brigitte; Barbat, Aline; Krausz, Pierre; Sainte-Catherine, Odile; Kraemer, Michel; Maes, Emmanuel; Guerardel, Yann (2010). "Structural Characterization and Cytotoxic Properties of an Apiose-Rich Pectic Polysaccharide Obtained from the Cell Wall of the Marine Phanerogam Zostera marina". Journal of Natural Products. 73 (6): 1087–1092. doi:10.1021/np100092c. PMID 20465284.
- Lv, Youjing; Shan, Xindi; Zhao, Xia; Cai, Chao; Zhao, Xiaoliang; Lang, Yinzhi; Zhu, He; Yu, Guangli (2015). "Extraction, Isolation, Structural Characterization and Anti-Tumor Properties of an Apigalacturonan-Rich Polysaccharide from the Sea Grass Zostera caespitosa Miki". Marine Drugs. 13 (6): 3710–3731. doi:10.3390/md13063710. PMC 4483652. PMID 26110894.
- Johnson, Kim L.; Jones, Brian J.; Bacic, Antony; Schultz, Carolyn J. (2003). "The Fasciclin-Like Arabinogalactan Proteins of Arabidopsis. A Multigene Family of Putative Cell Adhesion Molecules". Plant Physiology. 133 (4): 1911–1925. doi:10.1104/pp.103.031237. PMC 300743. PMID 14645732.
- Ellis, Miriam; Egelund, Jack; Schultz, Carolyn J.; Bacic, Antony (2010). "Arabinogalactan-Proteins: Key Regulators at the Cell Surface?". Plant Physiology. 153 (2): 403–419. doi:10.1104/pp.110.156000. PMC 2879789. PMID 20388666.
- Ma, Yingxuan; Zeng, Wei; Bacic, Antony; Johnson, Kim (2018). "AGPs Through Time and Space". Annual Plant Reviews online. pp. 767–804. doi:10.1002/9781119312994.apr0608. ISBN 9781119312994. S2CID 104384164.
- Classen, Birgit; Baumann, Alexander; Utermoehlen, Jon (2019). "Arabinogalactan-proteins in spore-producing land plants". Carbohydrate Polymers. 210: 215–224. doi:10.1016/j.carbpol.2019.01.077. PMID 30732757. S2CID 73426733.
- Pfeifer, Lukas; Shafee, Thomas; Johnson, Kim L.; Bacic, Antony; Classen, Birgit (2020). "Arabinogalactan-proteins of Zostera marina L. Contain unique glycan structures and provide insight into adaption processes to saline environments". Scientific Reports. 10 (1): 8232. Bibcode:2020NatSR..10.8232P. doi:10.1038/s41598-020-65135-5. PMC 7237498. PMID 32427862.
- Lamport, Derek T. A.; Kieliszewski, Marcia J.; Showalter, Allan M. (2006). "Salt stress upregulates periplasmic arabinogalactan proteins: Using salt stress to analyse AGP function". New Phytologist. 169 (3): 479–492. doi:10.1111/j.1469-8137.2005.01591.x. PMID 16411951.
- Opsahl, S.; Benner, R. (1993). "Decomposition of senescent blades of the seagrass Halodule wrightii in a subtropical lagoon". Marine Ecology Progress Series. 94: 191–205. Bibcode:1993MEPS...94..191O. doi:10.3354/meps094191.
- Klap, VA; Hemminga, MA; Boon, JJ (2000). "Retention of lignin in seagrasses:angiosperms that returned to the sea". Marine Ecology Progress Series. 194: 1–11. Bibcode:2000MEPS..194....1K. doi:10.3354/meps194001.
- Martone, Patrick T.; Estevez, José M.; Lu, Fachuang; Ruel, Katia; Denny, Mark W.; Somerville, Chris; Ralph, John (2009). "Discovery of Lignin in Seaweed Reveals Convergent Evolution of Cell-Wall Architecture". Current Biology. 19 (2): 169–175. doi:10.1016/j.cub.2008.12.031. PMID 19167225. S2CID 17409200.
- Kaal, Joeri; Serrano, Oscar; Del Río, José C.; Rencoret, Jorge (2018). "Radically different lignin composition in Posidonia species may link to differences in organic carbon sequestration capacity". Organic Geochemistry. 124: 247–256. doi:10.1016/j.orggeochem.2018.07.017. S2CID 105104424.
- Duarte, Carlos M. (June 2002). "The future of seagrass meadows". Environmental Conservation. 29 (2): 192–206. doi:10.1017/S0376892902000127. hdl:10261/89840. ISSN 1469-4387. S2CID 31971900.
- Waycott, Michelle; Duarte, Carlos M.; Carruthers, Tim J. B.; Orth, Robert J.; Dennison, William C.; Olyarnik, Suzanne; Calladine, Ainsley; Fourqurean, James W.; Heck, Kenneth L.; Hughes, A. Randall; Kendrick, Gary A. (28 July 2009). "Accelerating loss of seagrasses across the globe threatens coastal ecosystems". Proceedings of the National Academy of Sciences. 106 (30): 12377–12381. Bibcode:2009PNAS..10612377W. doi:10.1073/pnas.0905620106. ISSN 0027-8424. PMC 2707273. PMID 19587236.
- Short, Frederick T.; Polidoro, Beth; Livingstone, Suzanne R.; Carpenter, Kent E.; Bandeira, Salomão; Bujang, Japar Sidik; Calumpong, Hilconida P.; Carruthers, Tim J. B.; Coles, Robert G.; Dennison, William C.; Erftemeijer, Paul L. A. (1 July 2011). "Extinction risk assessment of the world's seagrass species". Biological Conservation. 144 (7): 1961–1971. doi:10.1016/j.biocon.2011.04.010. ISSN 0006-3207. S2CID 32533417.
- Orth, Robert J.; Carruthers, Tim J. B.; Dennison, William C.; Duarte, Carlos M.; Fourqurean, James W.; Heck, Kenneth L.; Hughes, A. Randall; Kendrick, Gary A.; Kenworthy, W. Judson; Olyarnik, Suzanne; Short, Frederick T. (1 December 2006). "A Global Crisis for Seagrass Ecosystems". BioScience. 56 (12): 987–996. doi:10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2. ISSN 0006-3568. S2CID 4936412.
- Burkholder, JoAnn M.; Tomasko, David A.; Touchette, Brant W. (9 November 2007). "Seagrasses and eutrophication". Journal of Experimental Marine Biology and Ecology. The Biology and Ecology of Seagrasses. 350 (1): 46–72. doi:10.1016/j.jembe.2007.06.024. ISSN 0022-0981.
- Telesca, Luca; Belluscio, Andrea; Criscoli, Alessandro; Ardizzone, Giandomenico; Apostolaki, Eugenia T.; Fraschetti, Simonetta; Gristina, Michele; Knittweis, Leyla; Martin, Corinne S.; Pergent, Gérard; Alagna, Adriana (28 July 2015). "Seagrass meadows (Posidonia oceanica) distribution and trajectories of change". Scientific Reports. 5 (1): 12505. Bibcode:2015NatSR...512505T. doi:10.1038/srep12505. ISSN 2045-2322. PMC 4516961. PMID 26216526.
- Unsworth, Richard K. F.; McKenzie, Len J.; Collier, Catherine J.; Cullen-Unsworth, Leanne C.; Duarte, Carlos M.; Eklöf, Johan S.; Jarvis, Jessie C.; Jones, Benjamin L.; Nordlund, Lina M. (1 August 2019). "Global challenges for seagrass conservation". Ambio. 48 (8): 801–815. doi:10.1007/s13280-018-1115-y. ISSN 1654-7209. PMC 6541581. PMID 30456457.
- Unsworth, Richard K. F.; Cullen-Unsworth, Leanne C.; Jones, Benjamin L.; Lilley, Richard J. (5 August 2022). "The planetary role of seagrass conservation". Science. 377 (6606): 609–613. Bibcode:2022Sci...377..609U. doi:10.1126/science.abq6923. PMID 35926055. S2CID 251347987.
- Xu, Shaochun; Qiao, Yongliang; Xu, Shuai; Yue, Shidong; Zhang, Yu; Liu, Mingjie; Zhang, Xiaomei; Zhou, Yi (1 June 2021). "Diversity, distribution and conservation of seagrass in coastal waters of the Liaodong Peninsula, North Yellow Sea, northern China: Implications for seagrass conservation". Marine Pollution Bulletin. 167: 112261. doi:10.1016/j.marpolbul.2021.112261. ISSN 0025-326X. PMID 33799145. S2CID 232775373.
- Newmaster, AF; Berg, KJ; Ragupathy, S.; Palanisamy, M.; Sambandan, K.; Newmaster, SG (23 November 2011). "Local Knowledge and Conservation of Seagrasses in the Tamil Nadu State of India". Journal of Ethnobiology and Ethnomedicine. 7 (1): 37. doi:10.1186/1746-4269-7-37. ISSN 1746-4269. PMC 3269989. PMID 22112297.
- Mohsin, Haroon (24 June 2022). "World Seagrass Day". National Today.
- "World Seagrass Day". World Seagrass Association. 10 June 2018. Retrieved 14 July 2022.
Further references
- den Hartog, C. 1970. The Sea-grasses of the World. Verhandl. der Koninklijke Nederlandse Akademie van Wetenschappen, Afd. Natuurkunde, No. 59(1).
- Duarte, Carlos M. and Carina L. Chiscano “Seagrass biomass and production: a reassessment” Aquatic Botany Volume 65, Issues 1–4, November 1999, Pages 159–174.
- Green, E.P. & Short, F.T.(eds). 2003. World Atlas of Seagrasses. University of California Press, Berkeley, CA. 298 pp.
- Hemminga, M.A. & Duarte, C. 2000. Seagrass Ecology. Cambridge University Press, Cambridge. 298 pp.
- Hogarth, Peter The Biology of Mangroves and Seagrasses (Oxford University Press, 2007)
- Larkum, Anthony W.D., Robert J. Orth, and Carlos M. Duarte (Editors) Seagrasses: Biology, Ecology and Conservation (Springer, 2006)
- Orth, Robert J. et al. "A Global Crisis for Seagrass Ecosystems" BioScience December 2006 / Vol. 56 No. 12, Pages 987–996.
- Short, F.T. & Coles, R.G.(eds). 2001. Global Seagrass Research Methods. Elsevier Science, Amsterdam. 473 pp.
- A.W.D. Larkum, R.J. Orth, and C.M. Duarte (eds). Seagrass Biology: A Treatise. CRC Press, Boca Raton, FL, in press.
- A. Schwartz; M. Morrison; I. Hawes; J. Halliday. 2006. Physical and biological characteristics of a rare marine habitat: sub-tidal seagrass beds of offshore islands. Science for Conservation 269. 39 pp.
- Waycott, M, McMahon, K, & Lavery, P 2014, A guide to southern temperate seagrasses, CSIRO Publishing, Melbourne
External links
- Cullen-Unsworth, Leanne C.; Unsworth, Richard (3 August 2018). "A call for seagrass protection". Science. 361 (6401): 446–448. Bibcode:2018Sci...361..446C. doi:10.1126/science.aat7318. ISSN 0036-8075. PMID 30072524. S2CID 51908021.
- Project Seagrass - Charity advancing the conservation of seagrass through community, research and action
- Seagrasses Project Regeneration.
- SeagrassSpotter - Citizen Science project raising awaress for seagrass meadows and mapping their locations
- Seagrass and Seagrass Beds overview from the Smithsonian Ocean Portal
- Nature Geoscience article describing the locations of the seagrass meadows around the world
- Seagrass-Watch - the largest scientific, non-destructive, seagrass assessment and monitoring program in the world
- Restore-A-Scar - a non-profit campaign to restore seagrass meadows damaged by boat props
- SeagrassNet - global seagrass monitoring program
- The Seagrass Fund at The Ocean Foundation
- Taxonomy of seagrasses
- World Seagrass Association
- SeagrassLI
- Seagrass Science and Management in the South China Sea and Gulf of Thailand
- Marine Ecology (December 2006) - special issue on seagrasses
- Cambodian Seagrasses
- Seagrass Productivity - COST Action ES0906
- Fisheries Western Australia - Seagrass Fact Sheet

