Rivaroxaban
Rivaroxaban, sold under the brand name Xarelto among others, is an anticoagulant medication (blood thinner) used to treat and prevent blood clots.[6] Specifically it is used to treat deep vein thrombosis and pulmonary emboli and prevent blood clots in atrial fibrillation and following hip or knee surgery.[6] It is taken by mouth.[6]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Xarelto, others |
| Other names | BAY 59-7939 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611049 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80–100%; Cmax = 2–4 hours (10 mg oral)[4] |
| Metabolism | CYP3A4, CYP2J2 and CYP-independent mechanisms[4] |
| Elimination half-life | 5–9 hours in healthy subjects aged 20 to 45[4][5] |
| Excretion | 2/3 metabolized in liver and 1/3 eliminated unchanged[4] |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.210.589 |
| Chemical and physical data | |
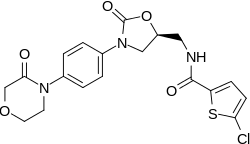
| Formula | C19H18ClN3O5S |
| Molar mass | 435.88 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Common side effects include bleeding.[6] Other serious side effects may include spinal hematoma and anaphylaxis.[6] It is unclear if use in pregnancy and breastfeeding is safe.[1] Compared to warfarin it has fewer interactions with other medications.[7] It works by blocking the activity of the clotting protein factor Xa.[6]
Rivaroxaban was patented in 2007 and approved for medical use in the United States in 2011.[8] In the United States, it will not be available as a generic medication until 2024.[9][10] It is on the World Health Organization's List of Essential Medicines.[11] In 2020, it was the 86th most commonly prescribed medication in the United States, with more than 8 million prescriptions.[12][13]
Medical uses
_tablets.jpg.webp)
In those with non-valvular atrial fibrillation, it appears to be as effective as warfarin in preventing ischemic strokes and embolic events.[14][15] Rivaroxaban is associated with lower rates of serious and fatal bleeding events than warfarin, though rivaroxaban is associated with higher rates of bleeding in the gastrointestinal tract.[16]
In July 2012, the UK's National Institute for Health and Clinical Excellence recommended rivaroxaban to prevent and treat venous thromboembolism.[17]
Contraindications
Because of the difficulty associated with managing bleeding, rivaroxaban should be discontinued at least 24 hours before surgery, then restarted as soon as adequate hemostasis is established.[18]
Dosing recommendations do not recommended administering rivaroxaban with drugs known to be strong combined CYP3A4/P-glycoprotein inhibitors because this results in significantly higher plasma concentrations of rivaroxaban.[2][19]
Adverse effects
The most serious adverse effect is bleeding, including severe internal bleeding.[20][21][22] Rivaroxaban is associated with lower rates of serious and fatal bleeding events than warfarin but is associated with higher rates of bleeding in the gastrointestinal tract.[16]
As of 2015, post-marketing assessments showed liver toxicity, and further studies are needed to quantify this risk.[23][24] In 2015, rivaroxaban accounted for the highest number of reported cases of serious injury among regularly monitored medications to the FDA's Adverse Events Reporting System (AERS).[25]
Reversal agent
In October 2014, Portola Pharmaceuticals completed Phase I and II clinical trials for andexanet alfa as an antidote for Factor Xa inhibitors with few adverse effects, and started Phase III trials.[26][27] Andexanet alfa was approved by the U.S. Food and Drug Administration in May 2018, under the trade name AndexXa.[28][29]
Mechanism of action
Rivaroxaban inhibits both free and bound Factor Xa in the prothrombinase complex.[30] It is a selective direct factor Xa inhibitor with an onset of action of 2.5 to 4 hours.[31] Inhibition of Factor Xa interrupts the intrinsic and extrinsic pathway of the blood coagulation cascade, inhibiting both thrombin formation and development of thrombi. Rivaroxaban does not inhibit thrombin (activated Factor II), and no effects on platelets have been demonstrated.[4] It allows predictable anticoagulation and dose adjustments and routine coagulation monitoring;[4] dietary restrictions are not needed.[32]
Unfractionated heparin (UFH), low molecular weight heparin (LMWH), and fondaparinux also inhibit the activity of factor Xa, indirectly, by binding to circulating antithrombin (AT III) and must be injected, whereas the orally active warfarin, phenprocoumon, and acenocoumarol are vitamin K antagonists (VKA), decreasing a number of coagulation factors, including factor X.[33]
Rivaroxaban has predictable pharmacokinetics across a wide spectrum of patients (age, gender, weight, race) and has a flat dose response across an eightfold dose range (5–40 mg).[34] The oral bioavailability is dose-dependent.[2] Doses of rivaroxaban under 10 mg can be taken with or without food, as it displayed high bioavailability independent of whether food was consumed or not.[35] If rivaroxaban is given at oral doses of 15 mg or 20 mg, it needs to be taken with food to aid in drug absorption and achieve appropriate bioavailability (≥ 80%).[35]
Chemistry
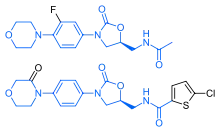
Rivaroxaban bears a striking structural similarity to the antibiotic linezolid: both drugs share the same oxazolidinone-derived core structure.[36] Accordingly, rivaroxaban was studied for any possible antimicrobial effects and for the possibility of mitochondrial toxicity, which is a known complication of long-term linezolid use.[37] Studies found that neither rivaroxaban nor its metabolites have any antibiotic effect against Gram-positive bacteria. As for mitochondrial toxicity, in vitro studies published before 2008 found the risk to be low.[36]
History
Rivaroxaban was initially developed by Bayer.[38] In the United States, it is marketed by Janssen Pharmaceuticals (a part of Johnson & Johnson).[38] It was the first available direct factor Xa inhibitor which is taken by mouth.[39]
Society and culture
Economics
Using rivaroxaban rather than warfarin costs 70 times more, according to Express Scripts Holding Co, the largest U.S. pharmacy benefits manager.[32] As of 2016, Bayer claimed that the drug was licensed in 130 countries and that more than 23 million patients had been treated.[40]
Approval
In September 2008, Health Canada granted marketing authorization for rivaroxaban to prevent venous thromboembolism (VTE) in people who have undergone elective total hip replacement or total knee replacement surgery.[41]
In the same month, the European Commission also granted marketing authorization of rivaroxaban to prevent venous thromboembolism in adults undergoing elective hip and knee replacement.[42][3]
On July 1, 2011, the United States Food and Drug Administration (US FDA) approved rivaroxaban for prophylaxis of deep vein thrombosis (DVT), which may lead to pulmonary embolism (PE), in adults undergoing hip and knee replacement surgery.[43]
On November 4, 2011, the US FDA approved rivaroxaban for stroke prevention in people with non-valvular atrial fibrillation.[44]
Legal action
On March 25, 2019, over 25,000 lawsuits over rivaroxaban in the US were settled for $775 million to get paid out to those affected. Plaintiffs accused the drugmakers of not warning about the bleeding risks, claiming their injuries could have been prevented had doctors and patients been provided adequate information.[45]
Research
Researchers at the Duke Clinical Research Institute have been accused of withholding clinical data used to evaluate rivaroxaban.[46] Duke tested rivaroxaban in a clinical trial known as the ROCKET AF trial.[47] The clinical trial, published 2011 in the New England Journal of Medicine[48] and headed by Robert Califf, then Commissioner of the FDA,[49][48] found rivaroxaban to be more effective than warfarin in reducing the likelihood of ischemic strokes in patients with atrial fibrillation.[48] The validity of the study was called into question in 2014 when pharmaceutical sponsors Bayer and Johnson & Johnson revealed that the INRatio blood monitoring devices used were not functioning properly,[46][47] A subsequent analysis by the Duke team published in February 2016 found that this had no significant effect on efficacy and safety in the trial.[50]
Under-representation of racial minorities in clinical trials has been noted. Compared to warfarin, efficacy and safety was found to be similar across racial subgroups.[48]
References
- "Rivaroxaban Use During Pregnancy". Drugs.com. Retrieved March 3, 2019.
- "Xarelto- rivaroxaban tablet, film coated Xarelto- rivaroxaban tablet, film coated Xarelto- rivaroxaban kit". DailyMed. Retrieved November 13, 2020.
- "Xarelto EPAR". European Medicines Agency (EMA). Retrieved November 13, 2020.
- "Xarelto: Summary of Product Characteristics". Bayer Schering Pharma AG. 2008. Retrieved February 11, 2009.
- Abdulsattar Y, Bhambri R, Nogid A (May 2009). "Rivaroxaban (xarelto) for the prevention of thromboembolic disease: an inside look at the oral direct factor xa inhibitor". P & T. 34 (5): 238–44. PMC 2697099. PMID 19561868.
- "Rivaroxaban Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved March 3, 2019.
- Kiser K (2017). Oral Anticoagulation Therapy: Cases and Clinical Correlation. Springer. p. 11. ISBN 9783319546438.
- "Generic Xarelto Availability". Drugs.com. Retrieved May 9, 2017.
- "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations". www.accessdata.fda.gov. Retrieved April 24, 2019.
- "Bayer, J&J Win Ruling That Upholds Patent for Xarelto Drug". April 22, 2019. Retrieved April 24, 2019.
- World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- "The Top 300 of 2020". ClinCalc. Retrieved October 7, 2022.
- "Rivaroxaban - Drug Usage Statistics". ClinCalc. Retrieved October 7, 2022.
- Lowenstern A, Al-Khatib SM, Sharan L, Chatterjee R, Allen LaPointe NM, Shah B, et al. (December 2018). "Interventions for Preventing Thromboembolic Events in Patients With Atrial Fibrillation: A Systematic Review". Annals of Internal Medicine. 169 (11): 774–787. doi:10.7326/M18-1523. PMC 6825839. PMID 30383133.
- Gómez-Outes A, Terleira-Fernández AI, Calvo-Rojas G, Suárez-Gea ML, Vargas-Castrillón E (2013). "Dabigatran, Rivaroxaban, or Apixaban versus Warfarin in Patients with Nonvalvular Atrial Fibrillation: A Systematic Review and Meta-Analysis of Subgroups". Thrombosis. 2013: 640723. doi:10.1155/2013/640723. PMC 3885278. PMID 24455237.
- Brown DG, Wilkerson EC, Love WE (March 2015). "A review of traditional and novel oral anticoagulant and antiplatelet therapy for dermatologists and dermatologic surgeons". Journal of the American Academy of Dermatology. 72 (3): 524–34. doi:10.1016/j.jaad.2014.10.027. PMID 25486915.
- "Overview | Rivaroxaban for the treatment of deep vein thrombosis and prevention of recurrent deep vein thrombosis and pulmonary embolism | Guidance | NICE". www.nice.org.uk. Retrieved January 1, 2020.
- Sunkara T, Ofori E, Zarubin V, Caughey ME, Gaduputi V, Reddy M (2016). "Perioperative Management of Direct Oral Anticoagulants (DOACs): A Systemic Review". Health Services Insights. 9 (Suppl 1): 25–36. doi:10.4137/HSI.S40701. PMC 5156547. PMID 28008269.
- Mueck W, Kubitza D, Becka M (September 2013). "Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects". British Journal of Clinical Pharmacology. 76 (3): 455–66. doi:10.1111/bcp.12075. PMC 3769672. PMID 23305158.
- "Medication Guide – Xarelto" (PDF). U.S. Food and Drug Administration. Retrieved September 1, 2014.
- "Xarelto Side Effects". WebMD. Retrieved September 1, 2014.
- "Xarelto Side Effects Center". RxList. Retrieved September 1, 2014.
- Raschi E, Poluzzi E, Koci A, Salvo F, Pariente A, Biselli M, et al. (August 2015). "Liver injury with novel oral anticoagulants: assessing post-marketing reports in the US Food and Drug Administration adverse event reporting system". British Journal of Clinical Pharmacology. 80 (2): 285–93. doi:10.1111/bcp.12611. PMC 4541976. PMID 25689417.
- Russmann S, Niedrig DF, Budmiger M, Schmidt C, Stieger B, Hürlimann S, Kullak-Ublick GA (August 2014). "Rivaroxaban postmarketing risk of liver injury" (PDF). Journal of Hepatology. 61 (2): 293–300. doi:10.1016/j.jhep.2014.03.026. PMID 24681117.
- Schroeder C. "ISMP Ranks Xarelto Most Dangerous Drug in the United States". DrugNews. DrugNews. Retrieved August 10, 2016.
- Schroeder C. "Possible Antidote Could Help Blood Thinner Patients In Bleeding Emergencies". DrugNews. Retrieved August 20, 2015.
- Mo Y, Yam FK (February 2015). "Recent advances in the development of specific antidotes for target-specific oral anticoagulants". Pharmacotherapy. 35 (2): 198–207. doi:10.1002/phar.1532. PMID 25644580. S2CID 22593448.
- "Accelerated Approval for AndexXa" (PDF). FDA. Retrieved August 1, 2018.
- "U.S. FDA Approves Portola Pharmaceuticals' Andexxa®, First and Only Antidote for the Reversal of Factor Xa Inhibitors". Portola Pharmaceuticals Inc. GlobeNewswire News Room. Retrieved August 1, 2018.
- Roehrig S, Straub A, Pohlmann J, Lampe T, Pernerstorfer J, Schlemmer KH, et al. (September 2005). "Discovery of the novel antithrombotic agent 5-chloro-N-({(5S)-2-oxo-3- [4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl}methyl)thiophene- 2-carboxamide (BAY 59-7939): an oral, direct factor Xa inhibitor". Journal of Medicinal Chemistry. 48 (19): 5900–8. doi:10.1021/jm050101d. PMID 16161994.
- Ansell J (January 2019). "Outpatient Oral Anticoagulant Therapy". Consultative Hemostasis and Thrombosis (Fourth ed.). Elsevier. pp. 747–777. doi:10.1016/B978-0-323-46202-0.00037-6. ISBN 978-0-323-46202-0.
- Berkrot B (December 23, 2015). "New blood thinner 'antidote' to help doctors move past warfarin". Reuters.
- Turpie AG (January 2008). "New oral anticoagulants in atrial fibrillation". European Heart Journal. 29 (2): 155–65. doi:10.1093/eurheartj/ehm575. PMID 18096568.
- Eriksson BI, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, et al. (November 2006). "A once-daily, oral, direct Factor Xa inhibitor, rivaroxaban (BAY 59-7939), for thromboprophylaxis after total hip replacement". Circulation. 114 (22): 2374–2381. doi:10.1161/CIRCULATIONAHA.106.642074. PMID 17116766.
- Stampfuss J, Kubitza D, Becka M, Mueck W (July 2013). "The effect of food on the absorption and pharmacokinetics of rivaroxaban". International Journal of Clinical Pharmacology and Therapeutics. 51 (7): 549–561. doi:10.5414/CP201812. PMID 23458226.
- European Medicines Agency (2008). "CHP Assessment Report for Xarelto (EMEA/543519/2008)" (PDF). Retrieved June 11, 2009.
- Singh AK, Noronha V, Gupta A, Singh D, Singh P, Singh A, Singh A. (2020). "Rivaroxaban: Drug review". Cancer Res Stat Treat. 3: 264–269. doi:10.4103/CRST.CRST_122_19.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - "Xarelto FDA Approval History". September 7, 2020.
- Fassiadis N (December 2009). "Rivaroxaban, the first oral, direct factor Xa inhibitor". Expert Opinion on Pharmacotherapy. 10 (18): 2945–2946. doi:10.1517/14656560903413559. PMID 19925048. S2CID 23498967.
- "Bayer comments on article in The British Medical Journal (BMJ) regarding Xarelto" (PDF). Bayer AG Communications, Government Relations & Corporate Brand. September 29, 2016.
- "Bayer's Xarelto Approved in Canada" (Press release). Bayer. September 16, 2008. Retrieved January 31, 2010.
- "Bayer's Novel Anticoagulant Xarelto now also approved in the EU" (Press release). Bayer. February 10, 2008. Retrieved January 31, 2010.
- "FDA Approves Xarelto® (rivaroxaban tablets) to Help Prevent Deep Vein Thrombosis in Patients Undergoing Knee or Hip Replacement Surgery" (Press release). Janssen Pharmaceutica. July 1, 2011. Archived from the original on November 5, 2011. Retrieved July 1, 2011.
- "FDA approves Xarelto to prevent stroke in people with common type of abnormal heart rhythm". US Food and Drug Association. November 4, 2011. Retrieved April 27, 2016.
- "Bayer, Johnson & Johnson settle more than 25,000 lawsuits over blood thinner Xarelto for $775 million". washingtonpost.com. Retrieved April 7, 2019.
- Thomas K (March 1, 2016). "Document Claims Drug Makers Deceived a Top Medical Journal". The New York Times. Retrieved May 3, 2016.
- Patel V. "Duke clinical trial under scrutiny in drug case". The Chronicle. Duke Student Publishing Company.
- Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. (September 2011). "Rivaroxaban versus warfarin in nonvalvular atrial fibrillation". The New England Journal of Medicine. 365 (10): 883–91. doi:10.1056/NEJMoa1009638. PMC 3860773. PMID 21830957.
- "Meet Robert M. Califf, M.D., Commissioner of Food and Drugs". U.S. Food and Drug Administration. U.S. Food and Drug Administration. Retrieved May 3, 2016.
- Patel MR, Hellkamp AS, Fox KA (February 2016). "Point-of-Care Warfarin Monitoring in the ROCKET AF Trial" (PDF). The New England Journal of Medicine. 374 (8): 785–8. doi:10.1056/NEJMc1515842. hdl:20.500.11820/69b742f0-5b6d-4f54-93a5-98a1da909653. PMID 26839968.
External links
- "Rivaroxaban". Drug Information Portal. U.S. National Library of Medicine.