Atypical antipsychotic
The atypical antipsychotics (AAP), also known as second generation antipsychotics (SGAs) and serotonin–dopamine antagonists (SDAs),[1][2] are a group of antipsychotic drugs (antipsychotic drugs in general are also known as major tranquilizers and neuroleptics, although the latter is usually reserved for the typical antipsychotics) largely introduced after the 1970s and used to treat psychiatric conditions. Some atypical antipsychotics have received regulatory approval (e.g. by the FDA of the US, the TGA of Australia, the MHRA of the UK) for schizophrenia, bipolar disorder, irritability in autism, and as an adjunct in major depressive disorder.
| Atypical antipsychotic | |
|---|---|
| Drug class | |
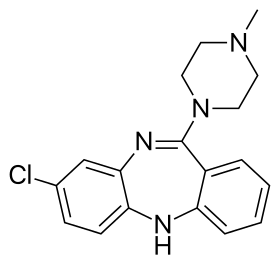 Skeletal formula of clozapine, the first atypical antipsychotic (1972) | |
| Synonyms | Second generation antipsychotic, serotonin–dopamine antagonist |
| In Wikidata | |
Both generations of medication tend to block receptors in the brain's dopamine pathways. Atypicals are less likely than haloperidol — the most widely used typical antipsychotic — to cause extrapyramidal motor control disabilities in patients such as unsteady Parkinson's disease-type movements, body rigidity, and involuntary tremors. However, only a few of the atypicals have been demonstrated to be superior to lesser-used, low-potency first-generation antipsychotics in this regard.[3][4][5]
As experience with these agents has grown, several studies have questioned the utility of broadly characterizing antipsychotic drugs as "atypical/second generation" as opposed to "first generation," noting that each agent has its own efficacy and side-effect profile. It has been argued that a more nuanced view in which the needs of individual patients are matched to the properties of individual drugs is more appropriate.[4][3] Although atypical antipsychotics are thought to be safer than typical antipsychotics, they still have severe side effects, including tardive dyskinesia (a serious movement disorder), neuroleptic malignant syndrome, and increased risk of stroke, sudden cardiac death, blood clots, and diabetes. Significant weight gain may occur. Critics have argued that "the time has come to abandon the terms first-generation and second-generation antipsychotics, as they do not merit this distinction."[6]
Medical uses
Atypical antipsychotics are typically used to treat schizophrenia or bipolar disorder.[7] They are also frequently used to treat agitation associated with dementia, anxiety disorder, autism spectrum disorder, and obsessive-compulsive disorder (an off-label use).[8] In dementia, they should only be considered after other treatments have failed and if the patient is a risk to themselves and/or others.[9]
Schizophrenia
The first-line psychiatric treatment for schizophrenia is antipsychotic medication,[10] which can reduce the positive symptoms of schizophrenia in about 8–15 days. Antipsychotics only appear to improve secondary negative symptoms of schizophrenia in the short term and may worsen negative symptoms overall.[11] Overall there is no good evidence that atypical antipsychotics have any therapeutic benefit for treating the negative symptoms of schizophrenia.[12]
There is very little evidence on which to base a risk and benefit assessment of using antipsychotics for long-term treatment.[13]
The choice of which antipsychotic to use for a specific patient is based on benefits, risks, and costs.[14] It is debatable whether, as a class, typical or atypical antipsychotics are better.[15] Both have equal drop-out and symptom relapse rates when typicals are used at low to moderate dosages.[16] There is a good response in 40–50% of patients, a partial response in 30–40%, and treatment resistance (failure of symptoms to respond satisfactorily after six weeks to two of three different antipsychotics) in the remaining 20%.[17] Clozapine is considered a first choice treatment for treatment resistant schizophrenia, especially in the short term; in the longer-terms the risks of adverse effects complicate the choice.[18] In turn, risperidone, olanzapine, and aripiprazole have been recommended for the treatment of first-episode psychosis.[19][20]
Efficacy in the treatment of schizophrenia
The utility of broadly grouping the antipsychotics into first generation and atypical categories has been challenged. It has been argued that a more nuanced view, matching the properties of individual drugs to the needs of specific patients is preferable.[3] While the atypical (second-generation) antipsychotics were marketed as offering greater efficacy in reducing psychotic symptoms while reducing side effects (and extrapyramidal symptoms in particular) than typical medications, the results showing these effects often lacked robustness, and the assumption was increasingly challenged even as atypical prescriptions were soaring.[21][22] In 2005 the US government body NIMH published the results of a major independent (not funded by the pharmaceutical companies) multi-site, double-blind study (the CATIE project).[23] This study compared several atypical antipsychotics to an older, mid-potency typical antipsychotic, perphenazine, among 1,493 persons with schizophrenia. The study found that only olanzapine outperformed perphenazine in discontinuation rate (the rate at which people stopped taking it due to its effects). The authors noted an apparent superior efficacy of olanzapine to the other drugs in terms of reduction in psychopathology and rate of hospitalizations, but olanzapine was associated with relatively severe metabolic effects such as a major weight gain problem (averaging 9.4 lbs over 18 months) and increases in glucose, cholesterol, and triglycerides. No other atypical studied (risperidone, quetiapine, and ziprasidone) did better than the typical perphenazine on the measures used, nor did they produce fewer adverse effects than the typical antipsychotic perphenazine (a result supported by a meta-analysis[3] by Leucht et al. published in The Lancet), although more patients discontinued perphenazine owing to extrapyramidal effects compared to the atypical agents (8% vs. 2% to 4%, P=0.002). A phase 2 part of this CATIE study roughly replicated these findings.[24] Compliance has not been shown to be different between the two types.[25] Overall evaluations of the CATIE and other studies have led many researchers to question the first-line prescribing of atypicals over typicals, or even to question the distinction between the two classes.[26][27][28]
It has been suggested that there is no validity to the term "second-generation antipsychotic drugs" and that the drugs that currently occupy this category are not identical to each other in mechanism, efficacy, and side-effect profiles. [29]
Each drug has its own mechanism, as RDr. if S. El-Mallakh, explained regarding the binding site and occupancy with a focus on the dopamine D2 receptor:
In general, when an antagonist of a neurotransmitter receptor is used, it must occupy a minimum of 65% to 70% of the target receptor to be effective. This is clearly the case when the target is a postsynaptic receptor, such as the dopamine D2 receptor. Similarly, despite significant variability in antidepressant response, blockade of 65% to 80% of presynaptic transport proteins—such as the serotonin reuptake pumps when considering serotoninergic antidepressants, or the norepinephrine reuptake pumps when considering noradrenergic agents such as nortriptyline—is necessary for these medications to be effective.... Depending on the level of intrinsic activity of a partial agonist and clinical goal, the clinician may aim for a different level of receptor occupancy. For example, aripiprazole will act as a dopamine agonist at lower concentrations, but blocks the receptor at higher concentrations. Unlike antagonist antipsychotics, which require only 65% to 70% D2 receptor occupancy to be effective, aripiprazole receptor binding at effective antipsychotic doses is 90% to 95%. Since aripiprazole has an intrinsic activity of approximately 30% (i.e, when it binds, it stimulates the D2 receptor to about 30% of the effect of dopamine binding to the receptor), binding to 90% of the receptors, and displacing endogenous dopamine, allows aripiprazole to replace the background or tonic tone of dopamine, which has been measured at 19% in people with schizophrenia and 9% in controls. Clinically, this still appears as the minimal effective dose achieving maximal response without significant parkinsonism despite >90% receptor occupancy.[30]
Bipolar disorder
In bipolar disorder, SGAs are most commonly used to rapidly control acute mania and mixed episodes, often in conjunction with mood stabilizers (which tend to have a delayed onset of action in such cases) such as lithium and valproate. In milder cases of mania or mixed episodes, mood stabilizer monotherapy may be attempted first.[31] SGAs are also used to treat other aspects of the disorder (such as acute bipolar depression or as a prophylactic treatment) as adjuncts or as a monotherapy, depending on the drug. Both quetiapine and olanzapine have demonstrated significant efficacy in all three treatment phases of bipolar disorder. Lurasidone (trade name Latuda) has demonstrated some efficacy in the acute depressive phase of bipolar disorder.[31][32][33]
Major depressive disorder
In non-psychotic major depressive disorder (MDD), some SGAs have demonstrated significant efficacy as adjunctive agents; and, such agents include:[34][35][36][37]
- Aripiprazole
- Brexpiprazole
- Olanzapine
- Quetiapine
- Ziprasidone[38]
whereas only quetiapine has demonstrated efficacy as a monotherapy in non-psychotic MDD.[39] Olanzapine/fluoxetine is an efficacious treatment in both psychotic and non-psychotic MDD.[40][41]
Aripiprazole, brexpiprazole, olanzapine, and quetiapine have been approved as adjunct treatment for MDD by the FDA in the United States.[42][43] Quetiapine and lurasidone have been approved, as monotherapies, for bipolar depression, but as of present, lurasidone has not been approved for MDD.[42]
Autism
Both risperidone and aripiprazole have received FDA approval for irritability in autism.[40]
Dementia and Alzheimer's disease
Between May 2007 and April 2008, Dementia and Alzheimer's together accounted for 28% of atypical antipsychotic use in patients aged 65 or older.[44] The U.S. Food and Drug Administration requires that all atypical antipsychotics carry a black box warning that the medication has been associated with an increased risk of mortality in elderly patients.[44] In 2005, the FDA issued an advisory warning of an increased risk of death when atypical antipsychotics are used in dementia.[45] In the subsequent 5 years, the use of atypical antipsychotics to treat dementia decreased by nearly 50%.[45]
Comparison table of efficacy
| Relative efficacy of SGAs | |||||
|---|---|---|---|---|---|
| Generic Drug Name[34][35][46][4][47] | Schizophrenia | Mania | Bipolar Maintenance | Bipolar Depression | Adjunct in Major Depressive Disorder |
| Amisulpride | +++ | ? | ? | ? | ? (+++ as a dysthymia monotherapy, however) |
| Aripiprazole | ++ | ++ | ++/+ | -[48] | +++ |
| Asenapine | +++ | ++ | ++ | ? (some evidence has suggested efficacy in treating depressive symptoms in mixed/manic episodes[49]) | ? |
| Blonanserin | ++ | ? | ? | ? | ? |
| Cariprazine | +++ | ? | ? | ? | ? |
| Clozapine | +++ | +++ | +++ | +++ | ? |
| Iloperidone | + | ? | ? | ? | ? |
| Lurasidone | + | ? | ? | +++[48] | ? |
| Melperone | +++/++ | ? | ? | ? | ? |
| Olanzapine | +++ | +++ | ++ | +++/++ (+++ when combined with fluoxetine)[48] | ++ |
| Paliperidone | ++ | ? | ? | ? | ? |
| Perospirone[50] | + | ? | ? | ? | ? |
| Quetiapine | ++ | ++ | +++ | ++[48] | ++ |
| Risperidone | +++ | +++ | ++ | -[48] | + |
| Sertindole | ++ | ? | ? | ? | ? |
| Ziprasidone | ++/+ | ++/+ | ? | -[48] | ? |
| Zotepine | ++ | ? | ? | ? | ? |
|
Legend:
| |||||
Adverse effects
The side effects reportedly associated with the various atypical antipsychotics vary and are medication-specific. Generally speaking, atypical antipsychotics are widely believed to have a lower likelihood for the development of tardive dyskinesia than the typical antipsychotics. However, tardive dyskinesia typically develops after long-term (possibly decades) use of antipsychotics. It is not clear if atypical antipsychotics, having been in use for a relatively short time, produce a lower incidence of tardive dyskinesia.[31][51]
Among the other side effects that have been suggested is that atypical antipsychotics increase the risk of cardiovascular disease.[52] Kabinoff et al. found that the increase in cardiovascular disease is seen regardless of the treatment received, and that it is instead caused by many different factors such as lifestyle or diet.[52]
Sexual side effects have also been reported when taking atypical antipsychotics.[53] In males antipsychotics reduce sexual interest, impair sexual performance with the main difficulties being failure to ejaculate.[54] In females there may be abnormal menstrual cycles and infertility.[55] In both males and females the breasts may become enlarged and a fluid will sometimes ooze from the nipples.[54] Sexual adverse effects caused by some anti-psychotics are a result of an increase of prolactin. Sulpiride and Amisulpiride, as well as Risperdone and paliperidone (to a lesser extent), cause a high increase of prolactin.
In April 2005, the US Food and Drug Administration (FDA) issued an advisory and subsequent black box warning regarding the risks of atypical antipsychotic use among elderly patients with dementia. The FDA advisory was associated with decreases in the use of atypical antipsychotics, especially among elderly patients with dementia.[56] Subsequent research reports confirmed the mortality risks associated with the use of both conventional and atypical antipsychotics to treat patients with dementia. Consequently, in 2008 the FDA issued although a black box warning for classical neuroleptics. Data on treatment efficacies are strongest for atypical antipsychotics. Adverse effects in patients with dementia include an increased risk of mortality and cerebrovascular events, as well as metabolic effects, extrapyramidal symptoms, falls, cognitive worsening, cardiac arrhythmia, and pneumonia.[57] Conventional antipsychotics may pose an even greater safety risk. No clear efficacy evidence exists to support the use of alternative psychotropic classes (e.g. antidepressants, anticonvulsants).[58]
Atypical antipsychotics may also cause anhedonia.[59]
Drug-induced OCD
Many different types of medication can induce in patients that have never had symptoms before. A new chapter about OCD in the DSM-5 (2013) now specifically includes drug-induced OCD.
There are reports that some atypical antipsychotics could cause drug-induced OCD in already schizophrenic patients.[60][61][62][63]
Tardive dyskinesia
All of the atypical antipsychotics warn about the possibility of tardive dyskinesia in their package inserts and in the PDR. It is not possible to truly know the risks of tardive dyskinesia when taking atypicals, because tardive dyskinesia can take many decades to develop and the atypical antipsychotics are not old enough to have been tested over a long enough period of time to determine all of the long-term risks. One hypothesis as to why atypicals have a lower risk of tardive dyskinesia is because they are much less fat-soluble than the typical antipsychotics and because they are readily released from D2 receptor and brain tissue.[64] The typical antipsychotics remain attached to the D2 receptors and accumulate in the brain tissue which may lead to TD.[64]
Both typical and atypical antipsychotics can cause tardive dyskinesia.[65] According to one study, rates are lower with the atypicals at 3.9% per year as opposed to the typicals at 5.5% per year.[65]
Metabolism
Recently, metabolic concerns have been of grave concern to clinicians, patients and the FDA. In 2003, the Food and Drug Administration (FDA) required all manufacturers of atypical antipsychotics to change their labeling to include a warning about the risks of hyperglycemia and diabetes with atypical antipsychotics. It must also be pointed out that although all atypicals must carry the warning on their labeling, some evidence shows that atypicals are not equal in their effects on weight and insulin sensitivity.[66] The general consensus is that clozapine and olanzapine are associated with the greatest effects on weight gain and decreased insulin sensitivity, followed by risperidone and quetiapine.[66] Ziprasidone and aripiprazole are thought to have the smallest effects on weight and insulin resistance, but clinical experience with these newer agents is not as developed as that with the older agents.[66] The mechanism of these adverse effects is not completely understood but it is believed to result from a complex interaction between a number of pharmacologic actions of these drugs. Their effects on weight are believed to mostly derive from their actions on the H1 and 5-HT2C receptors, while their effects on insulin sensitivity are believed to be the result of a combination of their effects on body weight (as increased body mass is known to be a risk factor for insulin resistance) and their antagonistic effects on the M3 receptor. Some of the newer agents, however, such as risperidone and its metabolite paliperidone, ziprasidone, lurasidone, aripiprazole, asenapine and iloperidone, have clinically insignificant effects on the M3 receptor and appear to carry a lower risk of insulin resistance. Whereas clozapine, olanzapine and quetiapine (indirectly via its active metabolite, norquetiapine) all antagonise the M3 receptor at therapeutic-relevant concentrations.[67]
Recent evidence suggests a role of the α1 adrenoceptor and 5-HT2A receptor in the metabolic effects of atypical antipsychotics. The 5-HT2A receptor, however, is also believed to play a crucial role in the therapeutic advantages of atypical antipsychotics over their predecessors, the typical antipsychotics.[68]
The two atypical antipsychotics with trials showing that had a low incidence of weight gain in large meta-analysis were lurasidone and aripiprazole.[69]
A study by Sernyak and colleagues found that the prevalence of diabetes in atypical antipsychotic treatments was statistically significantly higher than that of conventional treatment.[52] The authors of this study suggest that it is a causal relationship the Kabinoff et al. suggest the findings only suggest a temporal association.[52] Kabinoff et al. suggest that there is insufficient data from large studies to demonstrate a consistent or significant difference in the risk of insulin resistance during treatment with various atypical antipsychotics.[52]
Comparison table of adverse effects
| Comparison of side effects for atypical antipsychotics | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Generic Name | Weight gain | Metabolic Effects | EPS | High prolactin | Sedation | Hypotension / Orthostasis | QTc prolongation | Anti-ACh effects | Other adverse effects | |||||
| Amisulpride | + | + | + | ++ | - | - | +++ | - | Seizures, suicidal ideation | |||||
| Aripiprazole | 0-10%[70] | 0-10%[70] | 10-20%[70] | -[70] | 10-20%[70] | 0-10%[70] | - | - | Seizures (0.1-0.3%), anxiety, rhabdomyolysis, pancreatitis (<0.1%), agranulocytosis (<1%), leukopenia, neutropenia, suicidal ideation, angioedema (0.1-1%) | |||||
| Asenapine | 0-10%[70] | 20%[70] | 0-10%[70] | 0-10%[70] | 10-20%[70] | 0-10%[70] | + | - | Immune hypersensitivity reaction, angioedema, suicidal ideation | |||||
| Blonanserin | +/- | - | ++ | + | +/- | - | + | +/- | ||||||
| Clozapine | 20-30%[70] | 0-15%[70] | -[70] | -[70] | >30%[70] | 20-30%[70] | + | +++ | Seizures (3-5%), agranulocytosis (1.3%), leukopenia, pneumonia, respiratory arrest, angle-closure glaucoma, eosinophilia (1%), thrombocytopenia, Stevens–Johnson syndrome, myocarditis, erythema multiforme and abnormal peristalsis | |||||
| Iloperidone | 0-10%[70] | 0-10%[70] | 0-10%[70] | -[70] | 10-20%[70] | 0-10%[70] | ++ | - | Suicidal ideation (0.4-1.1%), syncope (0.4%) | |||||
| Lurasidone | -[70] | -[70] | >30%[70] | -[70] | 20-30%[70] | -[70] | + | + | Agranulocytosis, seizures (<1%), elevated serum creatinine (2-4%) | |||||
| Melperone | + | + | +/- | - | +/++ | +/++ | ++ | - | Agranulocytosis, neutropenia and leukopenia | |||||
| Olanzapine | 20-30%[70] | 0-15%[70] | 20-30%[70] | 20-30%[70] | >30%[70] | 0-10%[70] | + | + | Acute haemorrhagic pancreatitis, immune hypersensitivity reaction, seizures (0.9%), status epilepticus, suicidal ideation (0.1-1%) | |||||
| Paliperidone | 0-10%[70] | -[70] | 10-20%[70] | >30%[70] | 20-30%[70] | 0-10%[70] | +/- (7%) | - | Agranulocytosis, leukopenia, priapism, dysphagia, hyperprolactinaemia, sexual dysfunction[71] | |||||
| Perospirone | ? | ? | >30%[72] | + | + | + | ? | - | Insomnia in up to 23%,[72] CPK elevation[72] neuroleptic malignant syndrome[72] | |||||
| Quetiapine | 20-30%[70] | 0-15%[70] | 10-20%[70] | -[70] | >30%[70] | 0-10%[70] | ++ | + | Agranulocytosis, leukopenia, neutropenia (0.3%), anaphylaxis, seizures (0.05-0.5%), priapism, tardive dyskinesia (0.1-5%), suicidal ideation, pancreatitis, syncope (0.3-1%) | |||||
| Remoxipride[73] | +/- | - | - | -[64] | - | +/- | ? | - | There is a risk of aplastic anaemia risk which is what led to its removal from the market. | |||||
| Risperidone | 10-20%[70] | 0-10%[70] | 20-30%[70] | >30%[70] | >30%[70] | 0-10%[70] | + | - | Syncope (1%), pancreatitis, hypothermia, agranulocytosis, leukopenia, neutropenia, thrombocytopenia, hyperprolactinaemia, sexual dysfunction,[71] thrombotic thrombocytopenic purpura, cerebrovascular incident (<5%), tardive dyskinesia (<5%), priapism, neuroleptic malignant syndrome (<1%), gynecomastia, galactorrhea[74] | |||||
| Sertindole | ++ | +/- | - | ++ | - | +++ | +++ | - | - | |||||
| Sulpiride | + | + | + | +++ | - | +++ | + | - | Jaundice | |||||
| Ziprasidone | 0-10%[70] | 0-10%[70] | 0-10%[70] | -[70] | 20-30%[70] | 0-10%[70] | ++ | - | Syncope (0.6%), dysphagia (0.1-2%), bone marrow suppression, seizure (0.4%), priapism | |||||
Discontinuation
The British National Formulary recommends a gradual withdrawal when discontinuing antipsychotics to avoid acute withdrawal syndrome or rapid relapse.[75] Symptoms of withdrawal commonly include nausea, vomiting, and loss of appetite.[76] Other symptoms may include restlessness, increased sweating, and trouble sleeping.[76] Less commonly there may be a feeling of the world spinning, numbness, or muscle pains.[76] Symptoms generally resolve after a short period of time.[76]
There is tentative evidence that discontinuation of antipsychotics can result in psychosis.[77] It may also result in reoccurrence of the condition that is being treated.[78] Rarely tardive dyskinesia can occur when the medication is stopped.[76]
Pharmacology
Pharmacodynamics
The atypical antipsychotics integrate with the serotonin (5-HT), norepinephrine (α, β), and dopamine (DA) receptors in order to effectively treat schizophrenia.
D2 Receptor: Hyperactive dopaminergic activity on D2 receptors in the mesolimbic pathway is responsible for the positive symptoms of schizophrenia (hallucinations, delusions, paranoia). After taking an antipsychotic, antagonism of D2 receptors occurs throughout the entire brain, leading to a number of deleterious side effects from D2 receptor antagonism throughout the entire dopamine pathway system. It's not possible to affect D2 receptors only in the mesolimbic pathway,[79][Stahl AP Explained 1 - 1] but 5-HT2A receptor antagonism reverses these side effects to some extent.[Stahl AP Explained 1 - 2] Reducing D2 dopaminergic activity in the mesolimbic pathway also results in an anhedonic effect, reducing pleasure and motivation. In the mesocortical pathway to the DLPFC and VMPFC, endogenous D2 receptor dopamine activity is sometimes low in schizophrenia, resulting in cognitive, affective, and, broadly, the negative symptoms of schizophrenia. D2 receptor antagonism further compounds these problems. In the nigrostriatal pathway, D2 receptor antagonism results in extrapyramidal symptoms. If this antagonism occurs long enough, symptoms of EPS may become permanent, even if antipsychotic use is discontinued. In the tuberoinfundibular pathway, D2 receptor antagonism results in elevated prolactin. If prolactin levels become high enough, hyperprolactinaemia may occur, resulting in sexual dysfunction, weight gain, more rapid demineralization of bones, and possibly galactorrhea and amenorrhea.[Stahl AP Explained 1 - 1]
5-HT2A Receptor: When serotonin is released on to postsynaptic 5-HT2A receptors, the dopamine neuron is inhibited, thus acting as a brake on dopamine release.[Stahl AP Explained 1 - 2] This brake is disrupted through action of a 5-HT2A antagonist, which disinhibits the dopamine neuron, stimulating dopamine release. The result of this is that dopamine competes with antipsychotic D2 antagonistic action at D2 receptors, thereby reducing antagonistic binding there and eliminating or lowering D2 antagonistic effects in several pathways of the dopamine system.[Stahl AP Explained 1 - 2] In the nigrostratial pathway, it reduces EPS. In the tuberoinfundibular pathway, it reduces or eliminates prolactin elevation.[Stahl AP Explained 1 - 3] Dopamine release in the mesolimbic pathway from 5-HT2A antagonism does not appear to be as robust as in the other pathways of the dopamine system, thereby accounting for why atypical antipsychotics still retain part of their efficacy against the positive symptoms of schizophrenia through their D2 antagonism.[Stahl AP Explained 1 - 3] When 5-HT2A antagonistic agent particles occupy 5-HT2A receptors in the mesocortical pathway and in the prefrontal cortex, the negative symptoms of schizophrenia, affective symptoms, and cognitive deficits and abnormalities are treated and reduced.[Stahl AP Explained 1 - 3] Furthermore, 5-HT2A receptor antagonism blocks the serotonergic excitation of cortical pyramidal cells, reducing glutamate release, which in turn lowers hyperactive dopaminergic D2 receptor activity in the mesolimbic pathway, reducing or eliminating the positive symptoms of schizophrenia.[Stahl AP Explained 1 - 3][80][81]
Brexpiprazole, approved by the US FDA in 2015, has a similar binding profile to aripiprazole as a partial D2 agonist with moderate histamine binding, but with brexipiprazole has a higher affinity for serotonin receptor 5-HT2A
Some effects of 5-HT1A receptor activation include decreased aggressive behavior/ideation,[82] increased sociability, and decreased anxiety and depression. 5-HT2C activation blocks dopamine and inhibits norepinephrine release. Blockade of the 5-HT2C receptor increases serotonin, releasing norepinephrine and dopamine within the brain.[79] But neuronal reuptake of norepinephrine is limited sharply by some antipsychotics, e.g. ziprasidone. Increased norepinephrine can cause increased glucose(blood sugar) levels.[83][84][85] Increased blood sugar levels by increased norepinephrine causes hunger in many humans, which is why weight gain occurs with some antipsychotics if the norepinephrine is not inhibited.[86][87][88][89][90] Inhibition of norepinephrine stabilizes mood in humans.[91] 5-HT6 receptor antagonists improve cognition, learning, and memory.[92] The 5-HT7 receptor is very potent for the mitigation of bipolar conditions and also yields an antidepressant effect. The antipsychotics asenapine,[93] lurasidone,[94][95] risperidone,[96] and aripiprazole[97] are very potent at the 5-HT7 receptor. Antagonistic affinity for the H1 receptor also has an antidepressant effect. H1 antagonism blocks serotonin and norepinephrine reuptake. Patients with increased histamine levels have been observed to have lower serotonin levels.[98] However, the H1 receptor is linked to weight gain. To have partial agonism at the 5-HT1A receptor can yield absence of weight gain in an antipsychotic. This is very relevant for ziprasidone,[99][100] but it creates a risk for a prolonged QTc interval.[101][102] On the other hand, blockade of the 5-HT3 receptor removes the risk for a prolonged QTc interval,[94] but then creates a larger risk for weight gain. Relation to the 5-HT3 receptor increases caloric uptake and glucose,[103] which is seen in clozapine and olanzapine.[104][105] Other ways for dopamine to resolve is to have agonism at both the D2 receptor and 5-HT1A receptor, which normalizes the dopamine level in the brain. This occurs with cariprazine and aripiprazole.
Whether the anhedonic, loss of pleasure and motivation effect resulting from dopamine insufficiency or blockade at D2 receptors in the mesolimbic pathway, which is mediated in some part by antipsychotics (and despite dopamine release in the mesocortical pathway from 5-HT2A antagonism, which is seen in atypical antipsychotics), or the positive mood, mood stabilization, and cognitive improvement effect resulting from atypical antipsychotic serotonergic activity is greater for the overall quality of life effect of an atypical antipsychotic is a question that is variable between individual experience and the atypical antipsychotic(s) being used.[79]
Terms
Inhibition. Disinhibition: The opposite process of inhibition, the turning on of a biological function. Release: Causes the appropriate neurotransmitters to be discharged in vesicles into the synapse where they attempt to bind to and activate a receptor. Downregulation and Upregulation.
Binding profile
Note: Unless otherwise specified, the drugs below serve as antagonists/inverse agonists at the receptors listed.
| Generic Name[106] | D1 | D2 | D3 | D4 | 5-HT1A | 5-HT1B | 5-HT2A | 5-HT2C | 5-HT6 | 5-HT7 | α1A | α1 | α2 | M1 | M3 | H1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amisulpride | - | ++++ | ++++ | - | - | - | - | - | - | ++/+ | - | +/- | - | - | - | |
| Aripiprazole | + | ++++ (PA) | +++ (PA) | + (PA) | +++ (PA) | + | +++ | ++ (PA) | + | +++ (PA) | ++/+ | + | - | - | ++/+ | |
| Asenapine | +++ | +++ | ++++ | +++ | +++ (PA) | +++ | ++++ | ++++ | ++++ | ++++ | +++ | +++ | - | - | +++ | |
| Blonanserin | - | ++++ | ++++ | + | - | ? | +++ | + | + | +/- | + (RC) | + (RC) | + | ? | - | |
| Brexpiprazole | ++ | +++++ (PA) | ++++ (PA) | ++++ | +++++ (PA) | +++ | +++++ | +++ (PA) | +++ | ++++ | ++ | - | +++ | |||
| Cariprazine | ++++ (PA) | +++++ (PA) | ++++ (PA) | +++ | ++ | ++ | ++ | - | - | +++ | ||||||
| Clozapine | ++ | ++ | ++ | +++ | ++ (PA) | ++/+ | ++++ | ++++ | +++ | +++ | ++++ | +++ | ++++ | +++ | ++++ | |
| Iloperidone | + | +++ | +++ | ++ | + (PA) | + | +++ | + | ++ | + | ++++ | +++/++ | - | - | +++ | |
| Lurasidone | + | ++++ | ++ | ++ | +++ (PA) | ? | ++++ | +/- | ? | ++++ | - | +++/++ | - | - | - | |
| Melperone | ? | ++ | ++++ | ++ | + (PA) | ? | ++ | + | - | ++ | ++ | ++ | - | - | ++ | |
| Olanzapine | +++ | +++ | +++ | +++ | + (PA) | ++ | ++++ | +++ | +++ | ++ | ++ | ++ | ++++ | ++++ | ++++ | |
| Paliperidone | ++ | +++ | +++ | ++ | + (PA) | +++/++ | ++++ | + | - | ++++/+++ | +++ | +++ | - | - | +++/++ | |
| Quetiapine | + | ++/+ | ++/+ | + | ++/+ (PA) | + | + | + | ++ | +++/++ | ++++ | +++/++ | ++ | +++ | ++++ | |
| Risperidone | ++ | +++ | ++ | +++ | + (PA) | ++ | ++++ | ++ | - | +++/++ | ++ | +++/++ | ++ | - | - | ++ |
| Sertindole | ? | +++ | +++ | +++ | ++/+ (PA) | ++ | ++++ | ++++ | +++ | ++ | ++++/+++ | + | - | - | ++/+ | |
| Sulpiride | ? | ++++ | ++++ | +++ | - | - | - | - | - | - | - | - | - | - | - | |
| Ziprasidone | +++/++ | +++ | +++ | +++/++ | +++ (PA) | +++ (PA) | ++++ | +++(PA) | ++ | +++ | +++/++ | ++ | - | - | ++ | |
| Zotepine | +++/++ | +++ | ++++/+++ | +++ | ++ (PA) | +++ | ++++ | ++++ (RC) | ++++ | ++++/+++ | +++ | +++/++ | ++ (RC) | ++ (RC) | ++++ |
Legend:
| No Affinity or No Data | |
| - | Clinically Insignificant |
| + | Low |
| ++ | Moderate |
| +++ | High |
| ++++ | Very High |
| +++++ | Exceptionally High |
| PA | Partial Agonist |
| RC | Cloned Rat Receptor |
Pharmacokinetics
Atypical antipsychotics are most commonly administered orally.[54] Antipsychotics can also be injected, but this method is not as common.[54] They are lipid-soluble, are readily absorbed from the digestive tract, and can easily pass the blood–brain barrier and placental barriers.[54] Once in the brain, the antipsychotics work at the synapse by binding to the receptor.[107] Antipsychotics are completely metabolized in the body and the metabolites are excreted in urine.[108] These drugs have relatively long half-lives.[54] Each drug has a different half-life, but the occupancy of the D2 receptor falls off within 24 hours with atypical antipsychotics, while lasting over 24 hours for the typical antipsychotics.[64] This may explain why relapse into psychosis happens quicker with atypical antipsychotics than with typical antipsychotics, as the drug is excreted faster and is no longer working in the brain.[64] Physical dependence with these drugs is very rare.[54] However, if the drug is abruptly discontinued, psychotic symptoms, movement disorders, and sleep difficulty may be observed.[54] It is possible that withdrawal is rarely seen because the AAP are stored in body fat tissues and slowly released.[54]
| Pharmacokinetic parameters of available atypical antipsychotics[109][110][111] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Drug | Route(s) of Administration[Note 1] | Half-life (t1/2 in hours) | Volume of distribution (Vd in L/kg) | Protein binding | Excretion | Enzymes involved in metabolism | Bioavailability | Peak plasma time (h) | Cmax (ng/mL) |
| Amisulpride | Oral | 12 | 5.8 | 16% | Urine (50%), faeces (20%; 70% of this is as unchanged drug)[Note 2] | ? | 48% | Two peaks: 1 hr & 3-4 hrs post-oral dosing | 39±3 (1 hr), 54±4 (3-4 hrs) |
| Aripiprazole | Oral, intramuscular (including depot) | 75 (94 for active metabolite) | 4.9 | 99% | Faeces (55%), urine (25%) | CYP2D6 & CYP3A4 | 87% (Oral), 100% (IM) | 3-5 | ? |
| Asenapine | Sublingual | 24 | 20-25 | 95% | Urine (50%), faeces (40%) | CYP1A2 & UGT1A4 | 35% (sublingual), <2% (Oral) | 0.5-1.5 | 4 |
| Blonanserin[112] | Oral | 10.7 (single 4 mg dose), 12 (single 8 mg dose), 16.2 (single 12 mg dose), 67.9 (repeated bid dosing) | ? | >99.7% | Urine (59%), faeces (30%) | CYP3A4 | 84% (Oral) | <2 | 0.14 (single 4 mg dose), 0.45 (single 8 mg dose), 0.76 (single 12 mg dose), 0.57 (bid dosing) |
| Clozapine | Oral | 8 hours (single dosing), 12 (twice daily dosing) | 4.67 | 97% | Urine (50%), faeces (30%) | CYP1A2, CYP3A4, CYP2D6 | 50-60% | 1.5-2.5 | 102-771 |
| Iloperidone | Oral | ? | 1340-2800 | 95% | Urine (45-58%), faeces (20-22%) | CYP2D6 & CYP3A4 | 96% | 2-4 | ? |
| Lurasidone | Oral | 18 | 6173 | 99% | Faeces (80%), urine (9%) | CYP3A4 | 9-19% | 1-3 | ? |
| Melperone[113][114] | Oral, intramuscular | 3–4 (oral), 6 (IM) | 7–9.9 | 50% | Urine (70% as metabolites; 5–10.4% unchanged drug) | ? | 65% (tablet), 87% (IM), 54% (oral syrup) | 0.5–3 | 75–324 (repeated dosing) |
| Olanzapine | Oral, intramuscular (including depot) | 30 | 1000 | 93% | Urine (57%), faeces (30%) | CYP1A2, CYP2D6 | >60% | 6 (Oral) | ? |
| Paliperidone | Oral, intramuscular (including depot) | 23 (Oral) | 390-487 | 74% | Urine (80%), faeces (11%) | CYP2D6, CYP3A4 | 28% | 24 (Oral) | ? |
| Perospirone[72] | Oral | ? | ? | 92% | Urine (0.4% as unchanged drug) | ? | ? | 1.5 | 1.9-5.7 |
| Quetiapine | Oral | 6 (IR), 7 (XR) | 6-14 | 83% | Urine (73%), faeces (20%) | CYP3A4 | 100% | 1.5 (IR), 6 (XR) | ? |
| Risperidone | Oral, intramuscular (including depot) | 3 (EM) (oral), 20 (PM) (oral) | 1-2 | 90%, 77% (metabolite) | Urine (70%), faeces (14%) | CYP2D6 | 70% | 3 (EM), 17 (PM) | ? |
| Sertindole | Oral | 72 (55-90) | 20 | 99.5% | Urine (4%), faeces (46-56%) | CYP2D6 | 74% | 10 | ? |
| Ziprasidone | Oral, intramuscular | 7 (oral) | 1.5 | 99% | Faeces (66%), urine (20%) | CYP3A4 & CYP1A2 | 60% (Oral), 100% (IM) | 6-8 | ? |
| Zotepine[115][116] | Oral | 13.7-15.9 | 10-109 | 97% | Urine (17%) | ? | 7-13% | ? | ? |
|
Acronyms used: | |||||||||
| Medication | Brand name | Class | Vehicle | Dosage | Tmax | t1/2 single | t1/2 multiple | logPc | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Aripiprazole lauroxil | Aristada | Atypical | Watera | 441–1064 mg/4–8 weeks | 24–35 days | ? | 54–57 days | 7.9–10.0 | |
| Aripiprazole monohydrate | Abilify Maintena | Atypical | Watera | 300–400 mg/4 weeks | 7 days | ? | 30–47 days | 4.9–5.2 | |
| Bromperidol decanoate | Impromen Decanoas | Typical | Sesame oil | 40–300 mg/4 weeks | 3–9 days | ? | 21–25 days | 7.9 | [117] |
| Clopentixol decanoate | Sordinol Depot | Typical | Viscoleob | 50–600 mg/1–4 weeks | 4–7 days | ? | 19 days | 9.0 | [118] |
| Flupentixol decanoate | Depixol | Typical | Viscoleob | 10–200 mg/2–4 weeks | 4–10 days | 8 days | 17 days | 7.2–9.2 | [118][119] |
| Fluphenazine decanoate | Prolixin Decanoate | Typical | Sesame oil | 12.5–100 mg/2–5 weeks | 1–2 days | 1–10 days | 14–100 days | 7.2–9.0 | [120][121][122] |
| Fluphenazine enanthate | Prolixin Enanthate | Typical | Sesame oil | 12.5–100 mg/1–4 weeks | 2–3 days | 4 days | ? | 6.4–7.4 | [121] |
| Fluspirilene | Imap, Redeptin | Typical | Watera | 2–12 mg/1 week | 1–8 days | 7 days | ? | 5.2–5.8 | [123] |
| Haloperidol decanoate | Haldol Decanoate | Typical | Sesame oil | 20–400 mg/2–4 weeks | 3–9 days | 18–21 days | 7.2–7.9 | [124][125] | |
| Olanzapine pamoate | Zyprexa Relprevv | Atypical | Watera | 150–405 mg/2–4 weeks | 7 days | ? | 30 days | – | |
| Oxyprothepin decanoate | Meclopin | Typical | ? | ? | ? | ? | ? | 8.5–8.7 | |
| Paliperidone palmitate | Invega Sustenna | Atypical | Watera | 39–819 mg/4–12 weeks | 13–33 days | 25–139 days | ? | 8.1–10.1 | |
| Perphenazine decanoate | Trilafon Dekanoat | Typical | Sesame oil | 50–200 mg/2–4 weeks | ? | ? | 27 days | 8.9 | |
| Perphenazine enanthate | Trilafon Enanthate | Typical | Sesame oil | 25–200 mg/2 weeks | 2–3 days | ? | 4–7 days | 6.4–7.2 | [126] |
| Pipotiazine palmitate | Piportil Longum | Typical | Viscoleob | 25–400 mg/4 weeks | 9–10 days | ? | 14–21 days | 8.5–11.6 | [119] |
| Pipotiazine undecylenate | Piportil Medium | Typical | Sesame oil | 100–200 mg/2 weeks | ? | ? | ? | 8.4 | |
| Risperidone | Risperdal Consta | Atypical | Microspheres | 12.5–75 mg/2 weeks | 21 days | ? | 3–6 days | – | |
| Zuclopentixol acetate | Clopixol Acuphase | Typical | Viscoleob | 50–200 mg/1–3 days | 1–2 days | 1–2 days | 4.7–4.9 | ||
| Zuclopentixol decanoate | Clopixol Depot | Typical | Viscoleob | 50–800 mg/2–4 weeks | 4–9 days | ? | 11–21 days | 7.5–9.0 | |
| Note: All by intramuscular injection. Footnotes: a = Microcrystalline or nanocrystalline aqueous suspension. b = Low-viscosity vegetable oil (specifically fractionated coconut oil with medium-chain triglycerides). c = Predicted, from PubChem and DrugBank. Sources: Main: See template. | |||||||||
History
The first major tranquilizer or antipsychotic medication, chlorpromazine (Thorazine), a typical antipsychotic, was discovered in 1951 and introduced into clinical practice shortly thereafter. Clozapine (Clozaril), an atypical antipsychotic, fell out of favor due to concerns over drug-induced agranulocytosis. Following research indicating its effectiveness in treatment-resistant schizophrenia and the development of an adverse event monitoring system, clozapine re-emerged as a viable antipsychotic. According to Barker (2003), the three most-accepted atypical drugs are clozapine, risperidone, and olanzapine. However, he goes on to explain that clozapine is usually the last resort when other drugs fail. Clozapine can cause agranulocytosis (a decreased number of white blood cells), requiring blood monitoring for the patient. Despite the effectiveness of clozapine for treatment-resistant schizophrenia, agents with a more favorable side-effect profile were sought for widespread use. During the 1990s, olanzapine, risperidone, and quetiapine were introduced, with ziprasidone and aripiprazole following in the early 2000s. The atypical anti-psychotic paliperidone was approved by the FDA in late 2006.
The atypical antipsychotics have found favor among clinicians and are now considered to be first-line treatments for schizophrenia and are gradually replacing the typical antipsychotics. In the past, most researchers have agreed that the defining characteristics of atypical antipsychotics are the decreased incidence of extrapyramidal side effects (EPS)[127] and an absence of sustained prolactin elevation.[64]
The terminology can still be imprecise. The definition of "atypicality" was based upon the absence of extrapyramidal side effects, but there is now a clear understanding that atypical antipsychotics can still induce these effects (though to a lesser degree than typical antipsychotics).[128] Recent literature focuses more upon specific pharmacological actions and less upon categorization of an agent as "typical" or "atypical". There is no clear dividing line between the typical and atypical antipsychotics therefore categorization based on the action is difficult.[64]
More recent research is questioning the notion that second-generation antipsychotics are superior to first generation typical anti-psychotics. Using a number of parameters to assess quality of life, Manchester University researchers found that typical antipsychotics were no worse than atypical antipsychotics. The research was funded by the National Health Service (NHS) of the UK.[129] Because each medication (whether first or second generation) has its own profile of desirable and adverse effects, a neuropsychopharmacologist may recommend one of the older ("typical" or first generation) or newer ("atypical" or second generation) antipsychotics alone or in combination with other medications, based on the symptom profile, response pattern, and adverse effects history of the individual patient.
Society and culture
Between May 2007 and April 2008, 5.5 million Americans filled at least one prescription for an atypical antipsychotic.[44] In patients under the age of 65, 71% of patients were prescribed an atypical antipsychotic to treat Schizophrenia or Bipolar Disorder where this dropped to 38% in patients aged 65 or above.[44]
Regulatory status
| Regulatory status of second-generation antipsychotics (SGAs) as of July 2013 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Generic name | United States FDA approved | Canada HPFB approved[130] | Australia TGA approved[131] | Europe EMA approved[132] | Japan PMDA approved[133] | United Kingdom MHRA approved[134][135][132] | ||||||||
| Amisulpride | No | No | Yes | (some members) | No | Yes | ||||||||
| Aripiprazole | Yes | Yes | Yes | Yes | Yes | Yes | ||||||||
| Asenapine | Yes | Yes | Yes | Yes | Yes | Yes | ||||||||
| Blonanserin | No | No | No | No | Yes | No | ||||||||
| Carpipramine | No | No | No | No | Yes | No | ||||||||
| Clocapramine | No | No | No | No | Yes | No | ||||||||
| Clozapine | Yes | Yes | Yes | Yes | Yes | Yes | ||||||||
| Iloperidone | Yes | No | No | Refused | No | No | ||||||||
| Lurasidone | Yes | Yes | Yes | Yes | No | Yes | ||||||||
| Melperone | No | No | No | No | No | No | ||||||||
| Mosapramine | No | No | No | No | Yes | No | ||||||||
| Olanzapine | Yes | Yes | Yes | Yes | Yes | Yes | ||||||||
| Paliperidone | Yes | Yes | Yes | Yes | Yes | Yes | ||||||||
| Perospirone | No | No | No | No | Yes | No | ||||||||
| Pimavanserin | Yes | No | No | Investigational | No | (No) | ||||||||
| Quetiapine | Yes | Yes | Yes | Yes | Yes | Yes | ||||||||
| Remoxipride | No | No | No | Withdrawn | No | No | ||||||||
| Risperidone | Yes | Yes | Yes | Yes | Yes | Yes | ||||||||
| Sertindole | No | No | No | Yes | No | Yes | ||||||||
| Sulpiride | No | No | No | Yes | Yes | Yes | ||||||||
| Ziprasidone | Yes | Yes | Yes | (some members) | No | Yes | ||||||||
| Zotepine | No | No | No | No | Yes | No | ||||||||
Notes
- The route of administration in this category refers to the standard means of administration when the drug is being used in its capacity as an atypical antipsychotic, not for other purposes. For example, amisulpride can be administered intravenously as an antiemetic drug but this is not its standard route of administration when being used as an antipsychotic
- Note these values are from a study in of which amisulpride was intravenously administered
Stahl: AP Explained 1
References
- Miyake, N; Miyamoto, S; Jarskog, LF (October 2012). "New serotonin/dopamine antagonists for the treatment of schizophrenia: are we making real progress?". Clinical Schizophrenia & Related Psychoses. 6 (3): 122–33. doi:10.3371/CSRP.6.3.4. PMID 23006237.
- Sadock, Benjamin J. (2014). Kaplan & Sadock's Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. Sadock, Virginia A.,, Ruiz, Pedro (11th ed.). Philadelphia. p. 318. ISBN 978-1-60913-971-1. OCLC 881019573.
- Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM (January 2009). "Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis". Lancet. 373 (9657): 31–41. doi:10.1016/S0140-6736(08)61764-X. PMID 19058842. S2CID 1071537.
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. (September 2013). "Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis". Lancet. 382 (9896): 951–62. doi:10.1016/S0140-6736(13)60733-3. PMID 23810019. S2CID 32085212.
- "A roadmap to key pharmacologic principles in using antipsychotics". Primary Care Companion to the Journal of Clinical Psychiatry. 9 (6): 444–54. 2007. doi:10.4088/PCC.v09n0607. PMC 2139919. PMID 18185824.
- Tyrer P, Kendall T (January 2009). "The spurious advance of antipsychotic drug therapy". Lancet. 373 (9657): 4–5. doi:10.1016/S0140-6736(08)61765-1. PMID 19058841. S2CID 19951248.
- "Respiridone". The American Society of Health-System Pharmacists. Retrieved April 3, 2011.
- Maher AR, Maglione M, Bagley S, Suttorp M, Hu JH, Ewing B, et al. (September 2011). "Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis". JAMA. 306 (12): 1359–69. doi:10.1001/jama.2011.1360. PMID 21954480.
- American Geriatrics Society 2012 Beers Criteria Update Expert Panel (April 2012). "American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults". Journal of the American Geriatrics Society. 60 (4): 616–31. doi:10.1111/j.1532-5415.2012.03923.x. PMC 3571677. PMID 22376048.
- "Schizophrenia: Full national clinical guideline on core interventions in primary and secondary care" (PDF). Gaskell and the British Psychological Society. National Collaborating Centre for Mental Health. March 25, 2009. Retrieved November 25, 2009.
- Aleman A, Lincoln TM, Bruggeman R, Melle I, Arends J, Arango C, Knegtering H (August 2017). "Treatment of negative symptoms: Where do we stand, and where do we go?" (PDF). Schizophrenia Research. 186: 55–62. doi:10.1016/j.schres.2016.05.015. PMID 27293137. S2CID 4907333.
- Fusar-Poli P, Papanastasiou E, Stahl D, Rocchetti M, Carpenter W, Shergill S, McGuire P (July 2015). "Treatments of Negative Symptoms in Schizophrenia: Meta-Analysis of 168 Randomized Placebo-Controlled Trials". Schizophrenia Bulletin. 41 (4): 892–9. doi:10.1093/schbul/sbu170. PMC 4466178. PMID 25528757.
- Murray RM, Quattrone D, Natesan S, van Os J, Nordentoft M, Howes O, et al. (November 2016). "Should psychiatrists be more cautious about the long-term prophylactic use of antipsychotics?". The British Journal of Psychiatry (Submitted manuscript). 209 (5): 361–365. doi:10.1192/bjp.bp.116.182683. PMID 27802977.
- van Os J, Kapur S (August 2009). "Schizophrenia". Lancet. 374 (9690): 635–45. doi:10.1016/S0140-6736(09)60995-8. PMID 19700006. S2CID 208792724.
- Kane JM, Correll CU (2010). "Pharmacologic treatment of schizophrenia". Dialogues in Clinical Neuroscience. 12 (3): 345–57. doi:10.31887/DCNS.2010.12.3/jkane. PMC 3085113. PMID 20954430.
- Schultz SH, North SW, Shields CG (June 2007). "Schizophrenia: a review". American Family Physician. 75 (12): 1821–9. PMID 17619525.
- Smith T, Weston C, Lieberman J (August 2010). "Schizophrenia (maintenance treatment)". American Family Physician. 82 (4): 338–9. PMID 20704164.
- Siskind D, McCartney L, Goldschlager R, Kisely S (November 2016). "Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis". The British Journal of Psychiatry. 209 (5): 385–392. doi:10.1192/bjp.bp.115.177261. PMID 27388573.
- Robinson, Delbert G.; Gallego, Juan A.; John, Majnu; Petrides, Georgios; Hassoun, Youssef; Zhang, Jian-Ping; Lopez, Leonardo; Braga, Raphael J.; Sevy, Serge M.; Addington, Jean; Kellner, Charles H. (2015). "A Randomized Comparison of Aripiprazole and Risperidone for the Acute Treatment of First-Episode Schizophrenia and Related Disorders: 3-Month Outcomes". Schizophrenia Bulletin. 41 (6): 1227–1236. doi:10.1093/schbul/sbv125. ISSN 1745-1701. PMC 4601722. PMID 26338693.
- Gómez-Revuelta, Marcos; Pelayo-Terán, José María; Juncal-Ruiz, María; Vázquez-Bourgon, Javier; Suárez-Pinilla, Paula; Romero-Jiménez, Rodrigo; Setién Suero, Esther; Ayesa-Arriola, Rosa; Crespo-Facorro, Benedicto (April 23, 2020). "Antipsychotic Treatment Effectiveness in First Episode of Psychosis: PAFIP 3-Year Follow-Up Randomized Clinical Trials Comparing Haloperidol, Olanzapine, Risperidone, Aripiprazole, Quetiapine, and Ziprasidone". The International Journal of Neuropsychopharmacology. 23 (4): 217–229. doi:10.1093/ijnp/pyaa004. ISSN 1469-5111. PMC 7177160. PMID 31974576.
- Alexander GC, Gallagher SA, Mascola A, Moloney RM, Stafford RS (February 2011). "Increasing off-label use of antipsychotic medications in the United States, 1995-2008". Pharmacoepidemiology and Drug Safety. 20 (2): 177–84. doi:10.1002/pds.2082. PMC 3069498. PMID 21254289.
- Geddes J, Freemantle N, Harrison P, Bebbington P (December 2000). "Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis". BMJ. 321 (7273): 1371–6. doi:10.1136/bmj.321.7273.1371. PMC 27538. PMID 11099280.
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. (September 2005). "Effectiveness of antipsychotic drugs in patients with chronic schizophrenia". The New England Journal of Medicine. 353 (12): 1209–23. doi:10.1056/NEJMoa051688. PMID 16172203.
- Stroup TS, Lieberman JA, McEvoy JP, Swartz MS, Davis SM, Rosenheck RA, et al. (April 2006). "Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic". The American Journal of Psychiatry. 163 (4): 611–22. doi:10.1176/appi.ajp.163.4.611. PMID 16585435.
- Voruganti LP, Baker LK, Awad AG (March 2008). "New generation antipsychotic drugs and compliance behaviour". Current Opinion in Psychiatry. 21 (2): 133–9. doi:10.1097/YCO.0b013e3282f52851. PMID 18332660. S2CID 34935.
- Paczynski RP, Alexander GC, Chinchilli VM, Kruszewski SP (January 2012). "Quality of evidence in drug compendia supporting off-label use of typical and atypical antipsychotic medications". The International Journal of Risk & Safety in Medicine. 24 (3): 137–46. doi:10.3233/JRS-2012-0567. PMID 22936056.
- Owens DC (2008). "How CATIE brought us back to Kansas: A critical re-evaluation of the concept of atypical antipsychotics and their place in the treatment of schizophrenia". Advances in Psychiatric Treatment. 14: 17–28. doi:10.1192/apt.bp.107.003970.
- Fischer-Barnicol D, Lanquillon S, Haen E, Zofel P, Koch HJ, Dose M, Klein HE (2008). "Typical and atypical antipsychotics--the misleading dichotomy. Results from the Working Group 'Drugs in Psychiatry' (AGATE)". Neuropsychobiology. 57 (1–2): 80–7. doi:10.1159/000135641. PMID 18515977. S2CID 2669203.
- Whitaker R (2010). Anatomy of an Epidemic. Crown. p. 303. ISBN 978-0307452412.
- "Receptor occupancy and drug response: Understanding the relationship". www.mdedge.com. Retrieved October 14, 2022.
- Taylor D, Paton C, Kapur S (2012). The Maudsley Prescribing Guidelines (12th ed.). Informa Healthcare. pp. 12–152, 173–196, 222–235.
- Soreff S, McInnes LA, Ahmed I, Talavera F (August 5, 2013). "Bipolar Affective Disorder Treatment & Management". Medscape Reference. WebMD. Retrieved October 10, 2013.
- Post RM, Keck P (July 30, 2013). "Bipolar Disorder in adults: Maintenance treatment". UpToDate. Wolters Kluwer Health. Retrieved October 10, 2013.
- Komossa K, Depping AM, Gaudchau A, Kissling W, Leucht S (December 2010). "Second-generation antipsychotics for major depressive disorder and dysthymia". The Cochrane Database of Systematic Reviews (12): CD008121. doi:10.1002/14651858.CD008121.pub2. PMID 21154393.
- Spielmans GI, Berman MI, Linardatos E, Rosenlicht NZ, Perry A, Tsai AC (2013). "Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes". PLOS Medicine. 10 (3): e1001403. doi:10.1371/journal.pmed.1001403. PMC 3595214. PMID 23554581.
- Nelson JC, Papakostas GI (September 2009). "Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials". The American Journal of Psychiatry. 166 (9): 980–91. doi:10.1176/appi.ajp.2009.09030312. PMID 19687129.
- Research, Center for Drug Evaluation and. "Drug Approvals and Databases - Drug Trials Snapshots: REXULTI for the treatment of major depressive disorder". www.fda.gov. Retrieved October 18, 2018.
- Papakostas GI, Fava M, Baer L, Swee MB, Jaeger A, Bobo WV, Shelton RC (December 2015). "Ziprasidone Augmentation of Escitalopram for Major Depressive Disorder: Efficacy Results From a Randomized, Double-Blind, Placebo-Controlled Study". The American Journal of Psychiatry. 172 (12): 1251–8. doi:10.1176/appi.ajp.2015.14101251. PMC 4843798. PMID 26085041.
- Maneeton N, Maneeton B, Srisurapanont M, Martin SD (September 2012). "Quetiapine monotherapy in acute phase for major depressive disorder: a meta-analysis of randomized, placebo-controlled trials". BMC Psychiatry. 12: 160. doi:10.1186/1471-244X-12-160. PMC 3549283. PMID 23017200.
- Truven Health Analytics, Inc. DRUGDEX System (Internet) [cited 2013 Oct 10]. Greenwood Village, CO: Thomsen Healthcare; 2013.
- Rothschild AJ, Williamson DJ, Tohen MF, Schatzberg A, Andersen SW, Van Campen LE, et al. (August 2004). "A double-blind, randomized study of olanzapine and olanzapine/fluoxetine combination for major depression with psychotic features". Journal of Clinical Psychopharmacology. 24 (4): 365–73. doi:10.1097/01.jcp.0000130557.08996.7a. PMID 15232326. S2CID 36295165.
- Roberts RJ, Lohano KK, El-Mallakh RS (September 2016). "Antipsychotics as antidepressants". Asia-Pacific Psychiatry. 8 (3): 179–88. doi:10.1111/appy.12186. PMID 25963405. S2CID 24264818.
- "U.S. FDA Approves Otsuka and Lundbeck's REXULTI (Brexpiprazole) as Adjunctive Treatment for Adults with Major Depressive Disorder and as a Treatment for Adults with Schizophrenia | Discover Otsuka". Otsuka in the U.S. Retrieved October 18, 2018.
- Cascade E, Kalali AH, Cummings JL (July 2008). "Use of atypical antipsychotics in the elderly". Psychiatry. 5 (7): 28–31. PMC 2695730. PMID 19727265.
- Ventimiglia J, Kalali AH, Vahia IV, Jeste DV (November 2010). "An analysis of the intended use of atypical antipsychotics in dementia". Psychiatry. 7 (11): 14–7. PMC 3010964. PMID 21191528.
- Cipriani A, Barbui C, Salanti G, Rendell J, Brown R, Stockton S, et al. (October 2011). "Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis". Lancet. 378 (9799): 1306–15. doi:10.1016/S0140-6736(11)60873-8. PMID 21851976. S2CID 25512763.
- Bishara D, Taylor D (2009). "Asenapine monotherapy in the acute treatment of both schizophrenia and bipolar I disorder". Neuropsychiatric Disease and Treatment. 5: 483–90. doi:10.2147/ndt.s5742. PMC 2762364. PMID 19851515.
- Taylor DM, Cornelius V, Smith L, Young AH (December 2014). "Comparative efficacy and acceptability of drug treatments for bipolar depression: a multiple-treatments meta-analysis". Acta Psychiatrica Scandinavica. 130 (6): 452–69. doi:10.1111/acps.12343. PMID 25283309. S2CID 23324764.
- Szegedi A, Zhao J, van Willigenburg A, Nations KR, Mackle M, Panagides J (June 2011). "Effects of asenapine on depressive symptoms in patients with bipolar I disorder experiencing acute manic or mixed episodes: a post hoc analysis of two 3-week clinical trials". BMC Psychiatry. 11: 101. doi:10.1186/1471-244X-11-101. PMC 3152513. PMID 21689438.
- Kishi T, Iwata N (September 2013). "Efficacy and tolerability of perospirone in schizophrenia: a systematic review and meta-analysis of randomized controlled trials". CNS Drugs. 27 (9): 731–41. doi:10.1007/s40263-013-0085-7. PMID 23812802. S2CID 11543666.
- Stroup TS, Marder S, Stein MB (October 23, 2013). "Pharmacotherapy for schizophrenia: Acute and maintenance phase treatment". UpToDate. Wolters Kluwer. Retrieved October 10, 2013.
- Kabinoff GS, Toalson PA, Healey KM, McGuire HC, Hay DP (February 2003). "Metabolic Issues With Atypical Antipsychotics in Primary Care: Dispelling the Myths". Primary Care Companion to the Journal of Clinical Psychiatry. 5 (1): 6–14. doi:10.4088/PCC.v05n0103. PMC 353028. PMID 15156241.
- Uçok A, Gaebel W (February 2008). "Side effects of atypical antipsychotics: a brief overview". World Psychiatry. 7 (1): 58–62. doi:10.1002/j.2051-5545.2008.tb00154.x. PMC 2327229. PMID 18458771.
- McKim W (2007). Antipsychotics in Drugs and Behavior: An Introduction to Behavioral Pharmacology. Upper Saddle River, NJ.: Pearson Prentice Hall. pp. 241–60.
- McKim W (2007). Psychomotor Stimulants in Drugs and Behavior: An Introduction to Behavioral Pharmacology. Upper Saddle River, NJ.: Pearson Prentice Hall. pp. 241–60.
- Dorsey ER, Rabbani A, Gallagher SA, Conti RM, Alexander GC (January 2010). "Impact of FDA black box advisory on antipsychotic medication use". Archives of Internal Medicine. 170 (1): 96–103. doi:10.1001/archinternmed.2009.456. PMC 4598075. PMID 20065205.
- Gerhard T, Huybrechts K, Olfson M, Schneeweiss S, Bobo WV, Doraiswamy PM, et al. (July 2014). "Comparative mortality risks of antipsychotic medications in community-dwelling older adults". The British Journal of Psychiatry. 205 (1): 44–51. doi:10.1192/bjp.bp.112.122499. PMID 23929443.
- Steinberg M, Lyketsos CG (September 2012). "Atypical antipsychotic use in patients with dementia: managing safety concerns". The American Journal of Psychiatry. 169 (9): 900–6. doi:10.1176/appi.ajp.2012.12030342. PMC 3516138. PMID 22952071.
- "Anhedonia: Symptoms, Treatment, and More". Healthline. July 5, 2016.
- Alevizos, Basil; Papageorgiou, Charalambos; Christodoulou, George N. (September 1, 2004). "Obsessive-compulsive symptoms with olanzapine". The International Journal of Neuropsychopharmacology. 7 (3): 375–377. doi:10.1017/S1461145704004456. ISSN 1461-1457. PMID 15231024.
- Kulkarni, Gajanan; Narayanaswamy, Janardhanan C.; Math, Suresh Bada (January 1, 2012). "Olanzapine induced de-novo obsessive compulsive disorder in a patient with schizophrenia". Indian Journal of Pharmacology. 44 (5): 649–650. doi:10.4103/0253-7613.100406. ISSN 0253-7613. PMC 3480803. PMID 23112432.
- Lykouras, L.; Zervas, I. M.; Gournellis, R.; Malliori, M.; Rabavilas, A. (September 1, 2000). "Olanzapine and obsessive-compulsive symptoms". European Neuropsychopharmacology. 10 (5): 385–387. doi:10.1016/s0924-977x(00)00096-1. ISSN 0924-977X. PMID 10974610. S2CID 276209.
- Schirmbeck, Frederike; Zink, Mathias (March 1, 2012). "Clozapine-Induced Obsessive-Compulsive Symptoms in Schizophrenia: A Critical Review". Current Neuropharmacology. 10 (1): 88–95. doi:10.2174/157015912799362724. ISSN 1570-159X. PMC 3286851. PMID 22942882.
- Seeman P (February 2002). "Atypical antipsychotics: mechanism of action". Canadian Journal of Psychiatry. 47 (1): 27–38. doi:10.1177/070674370204700106. PMID 11873706.
- Correll CU, Schenk EM (March 2008). "Tardive dyskinesia and new antipsychotics". Current Opinion in Psychiatry. 21 (2): 151–6. doi:10.1097/YCO.0b013e3282f53132. PMID 18332662. S2CID 37288246.
- "Consensus development conference on antipsychotic drugs and obesity and diabetes". Diabetes Care. 27 (2): 596–601. February 2004. doi:10.2337/diacare.27.2.596. PMID 14747245.
- Brunton L, Chabner B, Knollman B (2010). Goodman and Gilman's The Pharmacological Basis of Therapeutics (12th ed.). McGraw Hill Professional. pp. 417–455.
- Guenette MD, Giacca A, Hahn M, Teo C, Lam L, Chintoh A, et al. (May 2013). "Atypical antipsychotics and effects of adrenergic and serotonergic receptor binding on insulin secretion in-vivo: an animal model". Schizophrenia Research. 146 (1–3): 162–9. doi:10.1016/j.schres.2013.02.023. PMID 23499243. S2CID 43719333.
- Ng-Mak, Daisy; Tongbram, Vanita; Ndirangu, Kerigo; Rajagopalan, Krithika; Loebel, Antony (August 1, 2018). "Efficacy and metabolic effects of lurasidone versus brexpiprazole in schizophrenia: a network meta-analysis". Journal of Comparative Effectiveness Research. 7 (8): 737–748. doi:10.2217/cer-2018-0016. ISSN 2042-6305. PMID 29697278. S2CID 13769615.
- "Comparison of Common Side Effects of Second Generation Atypical Antipsychotics". Facts & Comparisons. Wolters Kluwer Health. Retrieved March 31, 2012.
- Park YW, Kim Y, Lee JH (December 2012). "Antipsychotic-induced sexual dysfunction and its management". The World Journal of Men's Health. 30 (3): 153–9. doi:10.5534/wjmh.2012.30.3.153. PMC 3623530. PMID 23596605.
- Onrust SV, McClellan K (2001). "Perospirone". CNS Drugs. 15 (4): 329–37, discussion 338. doi:10.2165/00023210-200115040-00006. PMID 11463136.
- Holm AC, Edsman I, Lundberg T, Odlind B (June 1993). "Tolerability of remoxipride in the long term treatment of schizophrenia. An overview". Drug Safety. 8 (6): 445–56. doi:10.2165/00002018-199308060-00005. PMID 8329149. S2CID 43855244.
- "Risperdal, gynecomastia and galactorrhea in adolescent males". Allnurses.com. Retrieved September 30, 2016.
- Joint Formulary Committee, BMJ, ed. (March 2009). "4.2.1". British National Formulary (57 ed.). United Kingdom: Royal Pharmaceutical Society of Great Britain. p. 192. ISBN 978-0-85369-845-6.
Withdrawal of antipsychotic drugs after long-term therapy should always be gradual and closely monitored to avoid the risk of acute withdrawal syndromes or rapid relapse.
- Haddad P, Haddad PM, Dursun S, Deakin B (2004). Adverse Syndromes and Psychiatric Drugs: A Clinical Guide. OUP Oxford. pp. 207–216. ISBN 9780198527480.
- Moncrieff J (July 2006). "Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse". Acta Psychiatrica Scandinavica. 114 (1): 3–13. doi:10.1111/j.1600-0447.2006.00787.x. PMID 16774655. S2CID 6267180.
- Sacchetti E, Vita A, Siracusano A, Fleischhacker W (2013). Adherence to Antipsychotics in Schizophrenia. Springer Science & Business Media. p. 85. ISBN 9788847026797.
- Stahl S. Antipsychotics Explained 1 (PDF). University Psychiatry. Retrieved December 6, 2017.
- Stephen M. Stahl (March 27, 2008). Antipsychotics and Mood Stabilizers: Stahl's Essential Psychopharmacology . p. 105. ISBN 9780521886642. Retrieved September 30, 2016.
- Egerton A, Ahmad R, Hirani E, Grasby PM (November 2008). "Modulation of striatal dopamine release by 5-HT2A and 5-HT2C receptor antagonists: [11C]raclopride PET studies in the rat". Psychopharmacology. 200 (4): 487–96. doi:10.1007/s00213-008-1226-4. PMID 18597077. S2CID 11800154.
- de Boer SF, Koolhaas JM (December 2005). "5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis". European Journal of Pharmacology. 526 (1–3): 125–39. doi:10.1016/j.ejphar.2005.09.065. PMID 16310183.
- "norepinephrine". Cardiosmart.org. December 15, 2010. Retrieved September 30, 2016.
- Veves A, Malik RA (February 1, 2008). Diabetic Neuropathy: Clinical Management. p. 401. ISBN 9781597453110. Retrieved September 30, 2016.
- "Epinephrine and Norepinephrine". Boundless.com. Archived from the original on October 13, 2016. Retrieved September 30, 2016.
- "Cariprazine - Side Effects, Uses, Dosage, Overdose, Pregnancy, Alcohol". RxWiki.com. September 17, 2015. Retrieved September 30, 2016.
- "INVEGA Side Effects - Schizophrenia Treatment – INVEGA (paliperidone)". Invega.com. May 17, 2016. Archived from the original on August 17, 2016. Retrieved September 30, 2016.
- "Diabetes Update: Hunger is a Symptom". Diabetesupdate.blogspot.se. August 8, 2007. Retrieved September 30, 2016.
- "High & Low Blood Sugar". Healthvermont.gov. Retrieved September 30, 2016.
- "REXULTI (brexpiprazole) | Important Safety Information". Rexulti.com. Retrieved September 30, 2016.
- Stephen M. Stahl (March 27, 2008). Antipsychotics and Mood Stabilizers: Stahl's Essential Psychopharmacology . p. 172. ISBN 9780521886642. Retrieved September 30, 2016.
- King MV, Marsden CA, Fone KC (September 2008). "A role for the 5-HT(1A), 5-HT4 and 5-HT6 receptors in learning and memory". Trends in Pharmacological Sciences. 29 (9): 482–92. doi:10.1016/j.tips.2008.07.001. PMID 19086256.
- Shahid M, Walker GB, Zorn SH, Wong EH (January 2009). "Asenapine: a novel psychopharmacologic agent with a unique human receptor signature". Journal of Psychopharmacology. 23 (1): 65–73. doi:10.1177/0269881107082944. PMID 18308814. S2CID 206489515.
- "Pharmcology Reviews : 200603" (PDF). Accessdata.fda.gov. Retrieved September 30, 2016.
- Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, et al. (July 2010). "Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity". The Journal of Pharmacology and Experimental Therapeutics. 334 (1): 171–81. doi:10.1124/jpet.110.167346. PMID 20404009. S2CID 12893717.
- National Institute of Mental Health. PDSD Ki Database (Internet) [cited 2013 Aug 10]. ChapelHill (NC): University of North Carolina. 1998-2013. Available from: "PDSP Database - UNC". Archived from the original on November 8, 2013. Retrieved May 16, 2016.
- Hemmings HC, Egan TD (2013). Pharmacology and Physiology for Anesthesia: Foundations and Clinical . p. 209. ISBN 978-1437716795. Retrieved September 30, 2016.
- Margaret Jordan Halter (March 12, 2014). Varcarolis' Foundations of Psychiatric Mental Health Nursing: A Clinical . p. 61. ISBN 9781455728886. Retrieved September 30, 2016.
- Tasman A, Kay J, First MB, Lieberman JA, Riba M (March 30, 2015). Psychiatry, 2 Volume Set. John Wiley & Sons. ISBN 978-1-118-84547-9.
- "FDA : Psychopharmacological Drugs Advisory Committee : Briefing Document for ZELDOX CAPSULES (Ziprasidone HCl)" (PDF). Fda.gov. July 19, 2000. Retrieved September 30, 2016.
- Wouters W, Tulp MT, Bevan P (May 1988). "Flesinoxan lowers blood pressure and heart rate in cats via 5-HT1A receptors". European Journal of Pharmacology. 149 (3): 213–23. doi:10.1016/0014-2999(88)90651-6. PMID 2842163.
- Horiuchi J, McDowall LM, Dampney RA (November 2008). "Role of 5-HT(1A) receptors in the lower brainstem on the cardiovascular response to dorsomedial hypothalamus activation". Autonomic Neuroscience. 142 (1–2): 71–6. doi:10.1016/j.autneu.2008.06.004. PMID 18667366. S2CID 20878941.
- Weber S, Volynets V, Kanuri G, Bergheim I, Bischoff SC (December 2009). "Treatment with the 5-HT3 antagonist tropisetron modulates glucose-induced obesity in mice". International Journal of Obesity. 33 (12): 1339–47. doi:10.1038/ijo.2009.191. PMID 19823183.
- Gobbi G, Janiri L (December 1999). "Clozapine blocks dopamine, 5-HT2 and 5-HT3 responses in the medial prefrontal cortex: an in vivo microiontophoretic study". European Neuropsychopharmacology. 10 (1): 43–9. doi:10.1016/S0924-977X(99)00055-3. PMID 10647096. S2CID 24251712.
- Navari RM (January 2014). "Olanzapine for the prevention and treatment of chronic nausea and chemotherapy-induced nausea and vomiting". European Journal of Pharmacology. 722: 180–6. doi:10.1016/j.ejphar.2013.08.048. PMID 24157985.
- Roth BL, Driscol J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina. Archived from the original on November 8, 2013. Retrieved October 10, 2013.
- Culpepper, 2007
- McKim, 2007
- "Medscape Multispecialty – Home page". WebMD. Retrieved November 27, 2013.
- "Therapeutic Goods Administration – Home page". Department of Health (Australia). Retrieved November 27, 2013.
- "Daily Med – Home page". U.S. National Library of Medicine. Retrieved November 27, 2013.
- Deeks ED, Keating GM (January 2010). "Blonanserin: a review of its use in the management of schizophrenia". CNS Drugs. 24 (1): 65–84. doi:10.2165/11202620-000000000-00000. PMID 20030420.
- Product Information: Eunerpan(R), Melperonhydrochlorid. Knoll Deutschland GmbH, Ludwigshafen, 1995.
- Borgström L, Larsson H, Molander L (1982). "Pharmacokinetics of parenteral and oral melperone in man". European Journal of Clinical Pharmacology. 23 (2): 173–6. doi:10.1007/BF00545974. PMID 7140807. S2CID 36697288.
- Product Information: Nipolept(R), zotepine. Klinge Pharma GmbH, Munich, 1996.
- Tanaka O, Kondo T, Otani K, Yasui N, Tokinaga N, Kaneko S (February 1998). "Single oral dose kinetics of zotepine and its relationship to prolactin response and side effects". Therapeutic Drug Monitoring. 20 (1): 117–9. doi:10.1097/00007691-199802000-00021. PMID 9485566.
- Parent M, Toussaint C, Gilson H (1983). "Long-term treatment of chronic psychotics with bromperidol decanoate: clinical and pharmacokinetic evaluation". Current Therapeutic Research. 34 (1): 1–6.
- Jørgensen A, Overø KF (1980). "Clopenthixol and flupenthixol depot preparations in outpatient schizophrenics. III. Serum levels". Acta Psychiatrica Scandinavica. Supplementum. 279: 41–54. doi:10.1111/j.1600-0447.1980.tb07082.x. PMID 6931472.
- Reynolds JE (1993). "Anxiolytic sedatives, hypnotics and neuroleptics.". Martindale: The Extra Pharmacopoeia (30th ed.). London: Pharmaceutical Press. pp. 364–623.
- Ereshefsky L, Saklad SR, Jann MW, Davis CM, Richards A, Seidel DR (May 1984). "Future of depot neuroleptic therapy: pharmacokinetic and pharmacodynamic approaches". The Journal of Clinical Psychiatry. 45 (5 Pt 2): 50–9. PMID 6143748.
- Curry SH, Whelpton R, de Schepper PJ, Vranckx S, Schiff AA (April 1979). "Kinetics of fluphenazine after fluphenazine dihydrochloride, enanthate and decanoate administration to man". British Journal of Clinical Pharmacology. 7 (4): 325–31. doi:10.1111/j.1365-2125.1979.tb00941.x. PMC 1429660. PMID 444352.
- Young D, Ereshefsky L, Saklad SR, Jann MW, Garcia N (1984). Explaining the pharmacokinetics of fluphenazine through computer simulations. (Abstract.). 19th Annual Midyear Clinical Meeting of the American Society of Hospital Pharmacists. Dallas, Texas.
- Janssen PA, Niemegeers CJ, Schellekens KH, Lenaerts FM, Verbruggen FJ, van Nueten JM, et al. (November 1970). "The pharmacology of fluspirilene (R 6218), a potent, long-acting and injectable neuroleptic drug". Arzneimittel-Forschung. 20 (11): 1689–98. PMID 4992598.
- Beresford R, Ward A (January 1987). "Haloperidol decanoate. A preliminary review of its pharmacodynamic and pharmacokinetic properties and therapeutic use in psychosis". Drugs. 33 (1): 31–49. doi:10.2165/00003495-198733010-00002. PMID 3545764.
- Reyntigens AJ, Heykants JJ, Woestenborghs RJ, Gelders YG, Aerts TJ (1982). "Pharmacokinetics of haloperidol decanoate. A 2-year follow-up". International Pharmacopsychiatry. 17 (4): 238–46. doi:10.1159/000468580. PMID 7185768.
- Larsson M, Axelsson R, Forsman A (1984). "On the pharmacokinetics of perphenazine: a clinical study of perphenazine enanthate and decanoate". Current Therapeutic Research. 36 (6): 1071–88.
- Farah A (2005). "Atypicality of atypical antipsychotics". Primary Care Companion to the Journal of Clinical Psychiatry. 7 (6): 268–74. doi:10.4088/PCC.v07n0602. PMC 1324958. PMID 16498489.
- Weiden PJ (January 2007). "EPS profiles: the atypical antipsychotics are not all the same". Journal of Psychiatric Practice. 13 (1): 13–24. doi:10.1097/00131746-200701000-00003. PMID 17242588. S2CID 46319827.
- Jones PB, Barnes TR, Davies L, Dunn G, Lloyd H, Hayhurst KP, et al. (October 2006). "Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1)". Archives of General Psychiatry. 63 (10): 1079–87. doi:10.1001/archpsyc.63.10.1079. PMID 17015810.
- "Drug Product Database Online Query". Government of Canada, Health Canada, Public Affairs, Consultation and Regions Branch. April 25, 2012. Retrieved February 1, 2018.
- "Search the TGA website". Australian Government Department of Health, Therapeutic Goods Administration. Retrieved February 2, 2018.
- "European Medicines Agency: Search". European Medicines Agency. Retrieved February 2, 2018. Search
- "Search". Pharmaceuticals and Medical Devices Agency. Archived from the original on November 16, 2018. Retrieved February 2, 2018.
- "Medicines Information: SPC & PILs". Medicines and Healthcare products Regulatory Agency. Retrieved February 2, 2018.
- "Guidance: Antipsychotic medicines". Medicines & Healthcare products Regulatory Agency. August 25, 2005. Retrieved February 2, 2018.
Further reading
- Elmorsy E, Smith PA (May 2015). "Bioenergetic disruption of human micro-vascular endothelial cells by antipsychotics" (PDF). Biochemical and Biophysical Research Communications. 460 (3): 857–62. doi:10.1016/j.bbrc.2015.03.122. PMID 25824037.
- Simpson GM (September 2005). "Atypical antipsychotics and the burden of disease". The American Journal of Managed Care. 11 (8 Suppl): S235-41. PMID 16180961.
- New antipsychotic drugs carry risks for children (USA Today 2006)
- "NIMH Study to Guide Treatment Choices for Schizophrenia" (Press release). National Institute of Mental Health. September 19, 2005. Archived from the original on September 2, 2013. Retrieved August 18, 2013.